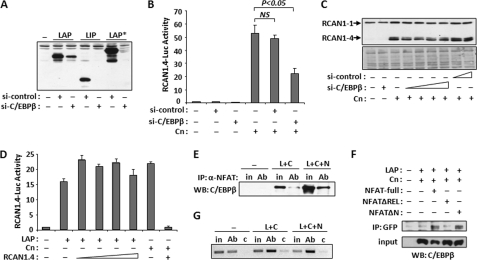
FIGURE 3.
C/EBPβ and NFATc1 form a complex and can bind the Rcan1–4 promoter. A, Western blot analysis showing depletion of LAP, LIP, and LAP* when cotransfected with a control (si-control) or C/EBPβ-specific (si-C/EBPβ) siRNA. B, luciferase activity was measured 24 h after co-transfection with RCAN1.4-Luc, si-control, si-C/EBPβ, and Cn. C, Western blot analysis for RCAN1 24 h after co-transfection with Cn and increasing amounts of si-control or si-C/EBPβ. Lower panel, Ponceau S staining of total protein. D, luciferase activity was assayed 24 h after transfection of C2C12 myoblasts with RCAN1.4-Luc, LAP, Cn, and increasing amounts of RCAN1.4 expression plasmid. E, C2C12 myoblasts were co-transfected with control vector (−), LAP and Cn (L+C), or LAP and Cn and NFATc1 (L+C+N) expression vectors. NFATc1-specific antibodies (Ab) were used for immunoprecipitation (IP), and the complexes were probed for the presence of C/EBPβ by Western blot (WB). Input samples (in) were run alongside for comparison (F). C2C12 myoblasts were transfected with plasmids encoding full-length NFATc1 fused to GFP (NFAT-full), amino acids 219–716 fused to GFP (NFATΔN) or 1–460 fused to GFP (NFATΔREL) along with C/EBPβ. Anti-GFP was used for co-immunoprecipitation and then probed for the presence of C/EBPβ. G, C2C12 myoblasts were transfected as in E. Protein-DNA complexes were precipitated with anti-C/EBPβ (Ab) or control IgG (c) and then assayed for the presence of the Rcan1–4 promoter by PCR. An input sample was used as a control. All experiments were carried out in triplicate. Error bars indicate S.D. NS, not significant.