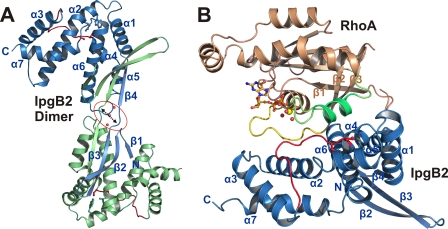
FIGURE 2.
Structures of dimeric IpgB2 and IpgB2·RhoA complex A in schematic representation. The conserved residues of the WXXXE motif, the coordinating double serine motif of the catalytic loop, and GDP are shown in ball and stick representation. A, domain-swapped dimer in free IpgB2. The two monomers are shown in blue and green, respectively, the catalytic loops are marked in red. Each N-terminal β-sheet domain separates from its C-terminal helical domain and is flipped over to the C-terminal domain of the respective other monomer. The hydrogen-bonding network at the interface between strands β3 and β4 of each monomer is disrupted by three water molecules (red spheres, marked by an arrow and a red circle), indicating that this dimer architecture is not physiologically relevant (see text). B, IpgB2·RhoA·GDP (complex A). IpgB2 is shown in blue, RhoA in brown. GDP is shown in orange and the GDP-coordinating Mg2+ is a yellow sphere. Red spheres are water molecules coordinating Mg2+. The switch I region is shown as a yellow loop, and the switch II is green. The catalytic loop of IpgB2 is shown as a red loop. The IpgB2 monomer in complex A corresponds to β2 and β3 from one monomer of free IpgB2 as shown in A plus β3 to the end of the second monomer.