Abstract
Studies evaluating the role of steroid hormones in sexual differentiation of the zebra finch song system have produced complicated and at times paradoxical results, and indicate that additional factors may be critical. Therefore, in a previous study we initiated a screen for differential gene expression in the telencephalon of developing male and female zebra finches. The use of cDNA microarrays and real-time quantitative PCR revealed increased expression of the genes encoding ribosomal proteins L17 and L37 (RPL17 and RPL37) in the male forebrain as a whole. Preliminary in situ hybridization data then indicated enhanced expression of both these genes in song control regions. Two experiments in the present study quantified the mRNA expression. The first utilized 25-day-old male and female zebra finches. The second compared a separate set of juveniles to adults of both sexes to both re-confirm enhanced expression in juvenile males and to determine whether it is limited to developing animals. In Experiment 1, males exhibited increased expression of both RPL17 and RPL37 compared to females in Area X, the robust nucleus of the arcopallium (RA), and the ventral ventricular zone (VVZ), which may provide neurons to Area X. Experiment 2 replicated the sexually dimorphic expression of these genes at post-hatching day 25, and documented that the sex differences are eliminated or greatly reduced in adults. The results are consistent with the idea that these ribosomal proteins may influence sexual differentiation of Area X and RA, potentially regulating the genesis and/or survival of neurons.
Keywords: Sexual differentiation, Masculinization, Ventricular zone, In situ hybridization, Songbird
1. Introduction
Sex differences in neural structure and function have been described in diverse vertebrate models. In many cases, they are permanently organized by exposure to gonadal hormones early in development (reviewed in Cooke et al., 1998; De Vries and Simerly, 2002). However, increasing evidence suggests roles for non-steroidal, genetic, factors (e.g., Arnold, 2002). This idea has become particularly relevant to the song system of zebra finches. In these birds, adult male sing, but females do not, and the forebrain regions that control song learning and production are far larger in males than in females, due in part to increased neuron number and soma size (reviewed in Wade, 2001). Exogenous estradiol can substantially, although not completely, masculinize the brain and behavior of females, and estrogen produced in the brain masculinizes at least one projection within the song circuit (Holloway and Clayton, 2001). However, the natural mechanisms controlling the increase in the volume of these structures, the size and number of neurons within them, and the capacity to display song are largely unknown (see Wade and Arnold, 2004).
To facilitate our understanding of these processes, we used cDNA microarrays to screen for differential gene expression in the forebrains of developing male and female zebra finches. The results were validated with real-time quantitative (q) PCR, and then in situ hybridization was used in a few animals to determine the brain regions in which they were expressed. Among others, genes encoding two ribosomal proteins (RP), RPL17 and RPL37, were clearly detected in the song control nuclei of juveniles, and their expression appeared in some cases to be increased in males compared to females (Wade et al., 2005).
The present studies were undertaken to characterize the expression of these genes more completely. In addition to completing the protein coding sequence, in Experiment 1 we used in situ hybridization on a larger sample of 25-day-old individuals to determine whether the expression of these genes was indeed increased in song control nuclei of males compared to females. This age is during the juvenile period when males are memorizing the songs of their fathers and when morphological differentiation of the song circuit is occurring (Nordeen and Nordeen, 1997; Doupe et al., 2004; Wade and Arnold, 2004). Experiment 2 was then designed to both provide an additional replication of male-biased mRNA expression at this developmental stage and to determine whether the sex difference is maintained into adulthood. If RPL17 or RPL37 is specific to the process of sexual differentiation, then one would predict diminished and/or sexually monomorphic expression in adulthood.
2. Results
2.1. Experiment 1
For both RPL17 and RPL37, main effects of sex (both F > 12.44; p < 0.006) and brain region (F > 8.07, p < 0.0001), as well as significant interactions between the two variables (F > 3.53, p < 0.015; Fig. 1) were detected. Specifically, expression of RPL17 was increased in males compared to females in Area X (p < 0.001), the ventral portion of the ventricular zone (VVZ; p = 0.001) which may contribute cells to Area X (see DeWulf and Bottjer, 2002; DeWulf and Bottjer, 2005), and probably the robust nucleus of the arcopallium (RA; p = 0.012, α = 0.010 for comparisons in 5 brain regions). In parallel, expression of RPL37 was greater in males than females in Area X (p = 0.001, α = 0.012 for comparisons within 4 brain regions), the VVZ (p < 0.001), and RA (p = 0.002).
Fig. 1.
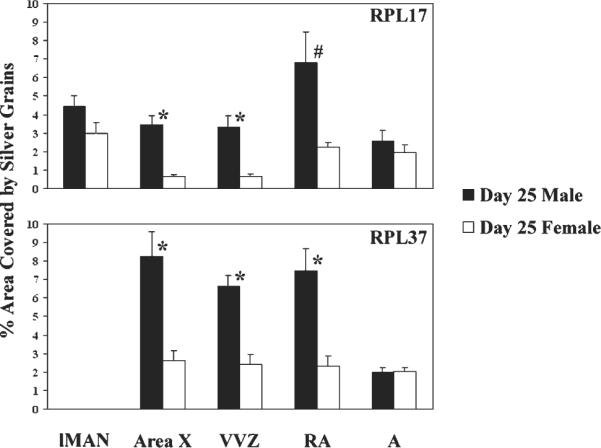
Experiment 1: Sex differences in the expression of genes encoding ribosomal protein L17 (RPL17; top) and RPL37 (bottom) in the song systems of 25-day-old zebra finches. In both cases, significant main effects of sex and brain region, as well as interactions between the two variables, were detected. Song control regions showing distinct labeling in a preliminary study (Wade et al., 2005) were evaluated, as was a control region outside of RA in the arcopallium (A). These areas were the same for the two genes, except for lMAN, in which we had detected RPL17 but not RPL37. * = significant effect of sex in pairwise planned comparisons (Bonferroni-corrected); # = one-tailed p value of 0.012 (α = 0.01 for 5 brain regions).
When the sexes were analyzed separately, both genes exhibited differences across brain regions within males (both F > 4.78, p < 0.008). For RPL17, mRNA in RA was increased compared to Area X, the VVZ, and the control region, A, (all p < 0.05) in males. In contrast, females (F = 13.09, p < 0.001) showed a decrease in RPL17 expression in Area X and the VVZ compared to A (p < 0.05). For RPL37, Area X, RA and the VVZ all showed increases compared to the control region, A, in males (all p < 0.05). However, no differences existed among the brain regions in females for this gene (F = 0.58, p = 0.635).
2.2. Experiment 2
For RPL17 (Figs. 2 and 3), significant main effects of age (F = 88.23, p < 0.0001) and sex (F = 78.65, p < 0.0001) were detected, as was a significant age × sex interaction (F = 18.53, p = 0.0003). An effect of brain region was also detected (F = 70.88, p < 0.0001), and all two- and three-way interactions involving this variable were statistically significant (all F > 5.63, p < 0.002). Within each of the regions of primary interest (RA, Area X and the VVZ), two-way ANOVAs revealed significant effects of age and sex, as well as interactions between them (all F > 6.00, p < 0.024). In the control region, A, a significant effect of only age was detected (F = 8.44, p = 0.009), and the magnitude of the difference between juveniles and adults was far smaller than in the other areas (Fig. 2). Planned, pairwise comparisons between sexes at 25 days of age (α = 0.0125 for 4 comparisons) revealed enhanced expression in males compared to females in Area X, RA and the VVZ (all t > 5.65, p ≤ 0.0002), but not A (t = 0.79, p = 0.939). In adults, the only significant sex difference was in RA (t =3.17, p = 0.010), and the magnitude of the dimorphism was far smaller than in juveniles (Fig. 2). Similarly, in males, all regions except A (t = 1.85, p = 0.095) showed increased expression in juveniles compared to adults (all t ≥ 6.05, p ≤ 0.0001), whereas in females RPL17 expression was statistically equivalent between the ages, although slightly higher in 25-day-old birds, in each of the four regions analyzed (all t < 2.33, all p > 0.042). For RPL37 (Figs. 2 and 3), all main effects and two- and three-way interactions were statistically significant (age: F = 95.45, p < 0.0001; sex: F = 123.37, p < 0.0001; age × sex: F = 73.05, p < 0.0001; brain region and all interactions with it: all F > 13.99; p < 0.0001). Two-way ANOVAs within each brain region except A (all F < 0.44, p > 0.519) revealed significant effects of age, sex, and interactions between these variables (all F > 25.86, p < 0.0001). Pairwise comparisons between the sexes at post-hatching day 25 detected enhanced expression in males for Area X, RA, and the VVZ (all t > 8.17, p < 0.0001). In contrast, sex differences were not detected in adults for the same regions (all t < 1.93, p ≥ 0.083). Among males, expression in Area X, RA, and the VVZ was increased in juveniles compared to adults (all t > 7.03, p < 0.0001), but not in A (t = 0.54, p = 0.600). In contrast, no differences in RPL37 expression were detected in any of the four brain regions in females (all t < 0.64, p > 0.541).
Fig. 2.
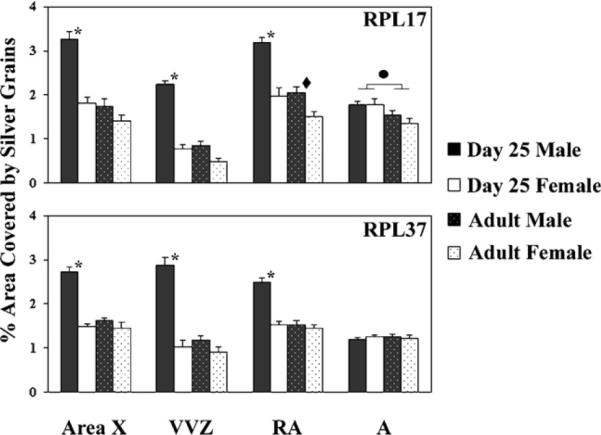
Experiment 2: Sex and age differences in the expression of genes encoding ribosomal protein L17 (RPL17; top) and RPL37 (bottom) in the song systems of juvenile and adult zebra finches. For both genes, main effects of sex, age, brain region, and all possible interactions among them were detected. * = increased expression in juvenile males compared to both juvenile females and adult males within a brain region. ◆ = increased expression in adult males compared to females in RA. ● = increased expression at 25 days of age compared to adulthood, including both sexes. All pairwise comparisons were Bonferroni-corrected.
Fig. 3.
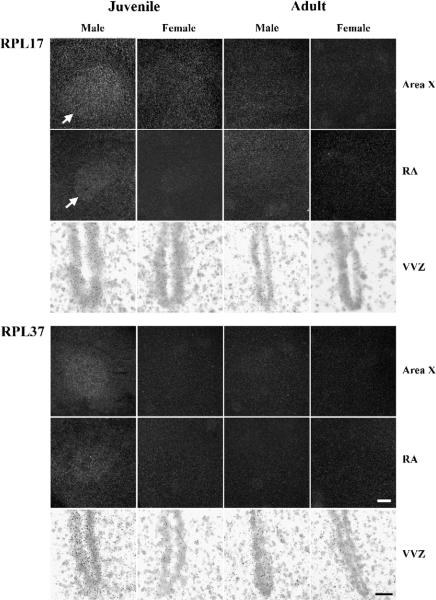
Experiment 2: Expression of mRNA encoding ribosomal proteins L17 (top) and L37 (bottom) in two song control nuclei (darkfield; Area X and the robust nucleus of the arcopallium, RA) and the ventral portion of the ventricular zone (brightfield; VVZ). Arrows indicate the ventromedial edge of Area X and RA for RPL17. Note the increased expression in these regions in juvenile males compared to all other groups, and the similar pattern for RPL37. Scale bars = 300 μm for all darkfield images and 30 μm for brightfield.
3. Discussion
The present results confirm the specific expression of the genes encoding two ribosomal proteins, RPL17 and RPL37, in the song system of developing male zebra finches. In Experiment 1, mRNA for both genes was significantly increased in males compared to females in Area X, RA, and the ventral portion of the ventricular zone in 25-day-old birds. The data from Experiment 2 replicate those results, and also clearly indicate that the sex differences are greatly diminished or no longer present in adulthood. Collectively, the results are consistent with the hypothesis that both RPL17 and RPL37 are involved in masculine development of the structure and/or function of two of the song control nuclei, and that their expression in the VVZ may be related to that process.
As indicated in the Introduction, we originally identified these genes in a screen for differential expression in the brains of developing males and females (Wade et al., 2005). That screen utilized cDNA microarrays followed by qPCR validation on cDNA from the entire forebrain. Then in situ hybridization was used on the brains of 25-day-old birds to determine anatomical localization. Only two individuals were investigated per sex with this technique, so these data were preliminary and based only on qualitative visual inspection of films and emulsion-coated slides. The present study was designed to quantify the mRNA expression, and overall the results agree quite well. The original work identified expression of RPL17 in the RA and ventricular epithelium of both sexes and in the Area X and lMAN of males only. RPL37 was seen in the ventricular zone of both sexes and in the Area X and RA of males only. The statistically significant sex differences detected in the forebrain as a whole using microarray and qPCR analyses could have come from any of these and/or other regions. We now can conclude that Area X, RA, and the VVZ all contributed, whereas lMAN probably did not. That is, while expression of RPL17 and RPL37 may be increased in juvenile males compared to females somewhat more broadly, the lack of sex differences detected in lMAN (for RPL17) and the area not related to song that was evaluated as a control (A; RPL17 and RPL37) indicate some specificity of the enhanced expression detected in Area X, RA, and the VVZ.
Very little is known about the function of these genes in any model system. However, levels of expression of ribosomal mRNAs are likely determined mainly by the rates of growth and proliferation of cells (Bévort and Leffers, 2000). These ideas are particularly consistent with the increased expression of RPL17 and RPL37 in the juvenile male RA and VVZ. The neurons in RA become larger in males than in females (Adkins-Regan et al., 1994), and the VVZ exhibits increased cell proliferation in juvenile males compared to females (DeWulf and Bottjer, 2005). Some data also suggest that, unlike the genes encoding most ribosomal proteins, RPL17 is up-regulated during early stages of retinoic acid induced differentiation of cultured human NTERA2 cells into neurons (Bévort and Leffers, 2000). It is therefore also possible that RPL17 influences the differentiation of neurons in Area X or RA.
The specific functions of RPL17 and RPL37 in song system differentiation still must be characterized, including a determination of whether the two genes serve parallel functions and/or interact with each other. However, the pattern of expression combined with what is known about mechanisms creating differences in cell number in song control nuclei allow us to generate some hypotheses. For example, in RA, cell death is increased in juvenile females compared to males, and appears to be at least partially independent of influences from lMAN and HVC, which project to it (reviewed in Wade and Arnold, 2004). Thus, the survival of neurons in males likely depends to some degree on the expression of factors intrinsic to RA. Sexual differentiation of Area X involves increased addition of neurons in males during post-hatching days 20–30 (Nordeen and Nordeen, 1988). DeWulf and Bottjer (2002; 2005) suggest that these cells may come from the VVZ at the rostrocaudal level of Area X, as this region exhibits enhanced cell division in one-month-old birds compared to adults, and among the juveniles a greater number of dividing cells exist in males compared to females. Thus, the present data documenting increased expression of RPL17 and RPL37 in males in all three of these brain regions, RA, Area X, and the VVZ, are all consistent with the idea that these genes (individually or in concert) influence cell survival during development, including those newly generated in the VVZ. It will be important in the future to narrow down the time course of their expression, and determine whether a critical period for their influence exists.
At this stage, we do not know whether the cells in Area X, RA, or the VVZ expressing RPL17 and/or RPL37 are neurons or glia (or their progenitors), or even whether the two mRNAs are in the same cells. It will be important to address these issues in future studies if we are to fully understand the mechanisms of any actions they may have in masculinizing the song circuit. For example, while as indicated above RPL17 and/or RPL37 may directly facilitate cell survival, it is also possible that the primary influence of these proteins involves cell proliferation. At least in RA, males show an increased number and percentage of glial cells on post-hatching day 30 that were generated a couple of days earlier (Nordeen and Nordeen, 1996). Trophic factors from these cells could inhibit neuron loss in males, as is the case when fibroblast growth factor 2 is infused into the RA of developing females (Nordeen et al., 1998). Thus, in addition to potentially acting in the VVZ to affect neurogenesis, it is possible that either or both RPL17 and RPL37 masculinize neuron number in Area X and RA in part by facilitating proliferation of glial cells that subsequently support a pattern of neuron survival more typical of developing males than females.
Questions also exist as to which stages of masculinization RPL17 and RPL37 may influence. For example, based on comparison to the chicken genome, RPL37 appears to be on a sex chromosome (the Z), whereas an obvious match for the location of RPL17 could not be determined (Wade et al., 2005). As male zebra finches have two Z-chromosomes and females only one (they are ZW; Itoh and Arnold, 2005), it is reasonable to hypothesize that RPL37 at least is involved in early stages of, or even initiating, masculinization. This idea will need to be tested, first by determining the location of both RPL17 and RPL37 on zebra finch chromosomes and then elucidating the specific actions of these genes by inhibiting their expression. While it might seem surprising that a gene encoding a ribosomal protein would be located on a sex chromosome, some precedent exists. In humans, RPS4X and RPS4Y both encode ribosomal protein S4. RPS4X is located on the X chromosome. It is not dosage compensated and escapes inactivation, yet appears to be expressed at comparable levels in human brain samples from the two sexes. These authors speculate that this result is due to the high degree of similarity between the X- and Y-forms of this gene (Vawter et al., 2004). In contrast, expression of the Y-chromosome form of RPS4Y, which is located in neurons, is strongly male-biased (Vawter et al., 2004).
Clearly, a substantial amount of work is required in order to understand the role of the proteins encoded by RPL17 and RPL37, including how they may interact with steroid hormones, which can masculinize both morphology of the song control nuclei and the capacity to display singing behavior in adulthood (reviewed in Wade and Arnold, 2004). Potential for these interactions is highlighted by the expression of androgen-related genes in the same brain areas. For example, during development, CYP17, which is required for androgen synthesis, is detected in the ventricular zone during development (London et al., 2003) and androgen receptors are expressed in Area X and RA, among other song control nuclei (Kim et al., 2004). Regardless of the details of how RPL17 and RPL37 may influence sexual differentiation, the fact that substantially enhanced expression of these genes in males is both specific to certain song control nuclei and limited to development strongly suggests that these genes are involved in the masculinization of the structure or function of these brain regions, and probably not in adult maintenance of these sexually dimorphic characteristics.
4. Experimental procedures
4.1. Tissue collection
As indicated above, two experiments were conducted. The first study used only juveniles, and was designed to determine the extent of the sex difference in gene expression at that developmental stage. The second experiment was designed to replicate those results using a different set of birds and to determine whether the sex differences were still exhibited in adulthood. All zebra finches were raised in our colony and rapidly decapitated. Experiment 1 used six individuals of each sex at post-hatching day 25 (day 1=the day of hatching) that had been reared in our colony at Michigan State University (MSU). Experiment 2 used additional 25-day-old birds that hatched at MSU, as well as adult males and females purchased from Magnolia Bird Farm (Anaheim, CA; n=6 in each of the 4 groups).
4.2. In situ hybridization
The brain was removed from each animal, frozen in cold methyl-butane, and stored at −80 °C. Brains were sectioned (20 μm) in the coronal plane and were thaw-mounted in six series onto SuperFrost Plus slides (Fisher Scientific, Hampton NH). They were stored with desiccant at −80 °C until further processing. In Experiment 1, two adjacent sets of tissue sections (one for antisense and one for sense probes) from each animal were used for in situ hybridization for each gene as described in Wade et al. (2005). As the background labeling with the sense probes for both RPL17 and RPL37 was very low, only antisense probes were used in Experiment 2. Within each experiment, all of the slides were hybridized simultaneously. The tissue sections were allowed to come to room temperature, rinsed in phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde, and washed in 0.1% diethylpyrocarbonate-treated water. Slides were rinsed again in PBS and incubated in 0.25% acetic anhydride in 0.1 M triethanolamine for 10 min, dehydrated, and air dried. They were pre-hybridized in a solution containing 50% formamide (buffer recipe in Wade et al., 2005) and then hybridized overnight at 55 °C with hybridization buffer, 10% dextran-sulfate, 50% formamide, and 5×106 cpm 33P-UTP-labeled RNA probe. Each probe was prepared using the MAXIscript In Vitro Transcription Kit with SP6/T7 RNA polymerases (Ambion, Austin, TX). After hybridization, slides were washed in SSC, treated with RNase A, washed again, dehydrated, and air dried. Slides were exposed to Hyperfilm MP (Amersham Biosciences, Piscataway, NJ) with an intensifying screen (BioMax Transcreen LE; Eastman Kodak, Rochester, NY) to confirm sufficient labeling, and then were dipped in emulsion (Eastman Kodak, Rochester, NY). NTB-2 was used in Experiment 1, but it was discontinued, so NTB was used for Experiment 2. The slides were developed 4 to 6 weeks later and lightly counter-stained with cresyl violet.
In Experiment 1, mRNA expression was quantified in each region related to the song system in which it was previously detected (Wade et al., 2005). Thus, RPL 17 was analyzed in lMAN, Area X, the VVZ, and RA. RPL37 was quantified in Area X, the VVZ and RA. When Area X was not visible (in females), a comparable region of the MSt was assessed. As a control, silver grains were also quantified in a portion of the lateral arcopallium (A) outside of RA, which is not involved in song learning, production, or perception. All analyses were done without knowledge of the sex of the individuals.
Within each brain region other than the VVZ (see below), images from both sides of each coronal section containing the area of interest (bilateral measurements in 3–6 sections per animal) were captured using Scion Image (NIH image) using darkfield microscopy. Due to the heavy nature of the labeling in some cases, as well as some scattering of silver grains, it was impossible to count silver grains over individual neurons. Thus, the area covered in a portion of each brain structure of interest was evaluated. The captured area was 264×198 μm2, which covered at least 50% of the cross-sectional area for each of the song control regions. As in Veney and Wade (2004, 2005), the density of labeling (percent area covered by silver grains) was quantified using the “density slice” function. For the VVZ, bilateral brightfield images were captured from the same sections in which Area X was analyzed, and the outline of approximately the ventral one-third of the VVZ was traced. The cross-sectional area was determined in this manner with Scion Image before quantifying the area covered by silver grains. For each brain region in this experiment, labeling in adjacent sense-treated sections was subtracted from the antisense values, and an average for each animal was calculated from the multiple measurements for use in statistical analyses.
Effects of sex (between individuals) and brain region (within individuals) on the percent area covered by silver grains were analyzed separately for each of the genes by two-way ANOVA. Based on the results (see above), these tests were followed by one-way ANOVAs within sex with Tukey/Kramer post hoc comparisons among brain regions. As previous work indicated both increased expression in song-related regions compared to others and increased expression in males compared to females (Wade et al., 2005), one-tailed pairwise comparisons were then used to further probe sexual dimorphisms within each of the brain areas, using Bonferroni corrections (adjusted α). All statistics were computed using Statview (SAS Institute, Cary, NC).
Data were collected in the same manner for Experiment 2 without knowledge of sex or age with two exceptions: RPL17 mRNA was not assessed in lMAN, since it did not show a sex difference in Experiment 1, and as sections were not exposed to sense probes, quantification of silver grains involved only averages of the percent area covered in antisense-treated tissue. In this study, a three-way ANOVA (sex and age as between subjects factors, and brain region within subjects) was used to analyze the data. Based on significant interactions, the calculations were broken down further as above, except that two-tailed tests were used for pairwise planned comparisons, as we had no previous data to suggest whether increased expression would be detected in adult males compared to females or whether it would differ in juveniles compared to adults within either sex.
4.3. Sequencing
The complete coding sequence of RPL17 (Genbank accession number AY833083), and partial sequence for RPL37 (AY833078) were reported previously (Wade et al., 2005). The remaining bases in the open reading frame of RPL37 were obtained using 5′ RACE. Telencephalic total RNA from a 25-day-old male zebra finch was extracted with Trizol (Invitrogen, Carlsbad CA) per manufacturer's instructions. It then was treated with RNase-free DNase (Qiagen, Valencia CA) during processing with an RNeasy MiniKit (Qiagen), followed by ethanol precipitation. The sample was then processed with the GeneRacer kit (Invitrogen) per manufacturer's instructions. First, cDNA was synthesized using the Platinum Pfx DNA Polymerase. Then, the manufacturer's PCR protocol was used with our gene-specific primer (5′-CTGGAGGCTGCAACAGCTGCTCTCTTGGGCTT-3′) to obtain sequence in the 5′ direction. PCR products were purified and cloned using the Zero Blunt TOPO PCR Cloning Kit for Sequencing (Invitrogen). Sequencing was accomplished using Big Dye Terminator v3.1 Cycle sequencing kit on a Prism 3100 genetic analyzer (Applied Biosystems, Foster City CA). The full-length sequence for RPL37 has been updated in Genbank (same accession number).
Acknowledgments
We are grateful to Camilla Peabody for technical assistance, in particular for obtaining the sequences of RPL17 and RPL37. This work was supported by NIH grants R01-MH55488 and K02-MH65907.
REFERENCES
- Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. Sexual differentiation of brain and behavior in the zebra finch: critical periods for effects of early estrogen treatment. J. Neurobiol. 1994;25:865–877. doi: 10.1002/neu.480250710. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol. 4. Academic Press; San Diego: 2002. pp. 105–135. [Google Scholar]
- Bévort M, Leffers H. Down regulation of ribosomal protein mRNAs during neuronal differentiation of human NTERA2 cells. Differentiation. 2000;66:81–92. doi: 10.1046/j.1432-0436.2000.660203.x. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and functions of sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. vol. 4. Academic Press; San Diego: 2002. pp. 137–191. [Google Scholar]
- DeWulf V, Bottjer SW. Age and sex differences in mitotic activity within the zebra finch telencephalon. J. Neurosci. 2002;22:4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone: a map of proliferative activity. J. Comp. Neurol. 2005;481:70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Solis MM, Kimpo R, Boettiger CA. Cellular, circuit, and synaptic mechanisms in song learning. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. Vol. 1016. New York Academy of Sciences; New York: 2004. pp. 495–523. [DOI] [PubMed] [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat. Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Arnold AP. Chromosomal polymorphism and comparative painting analysis in the zebra finch. Chromosome Res. 2005;13:47–56. doi: 10.1007/s10577-005-6602-x. [DOI] [PubMed] [Google Scholar]
- Kim Y-H, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J. Comp. Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: a study of its neural expression throughout posthatch development. J. Comp. Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. J. Neurosci. 1988;8:2869–2874. doi: 10.1523/JNEUROSCI.08-08-02869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Sex difference among nonneuronal cells precedes sexually dimorphic neuron growth and survival in an avian song control nucleus. J. Neurobiol. 1996;30:531–542. doi: 10.1002/(SICI)1097-4695(199608)30:4<531::AID-NEU8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Anatomical and synaptic substrates for avian song learning. J. Neurobiol. 1997;33:532–548. doi: 10.1002/(sici)1097-4695(19971105)33:5<532::aid-neu4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Voelkel L, Nordeen KW. Fibroblast growth factor-2 stimulates cell proliferation and decreases sexually dimorphic cell death in an avian song control nucleus. J. Neurobiol. 1998;37:573–581. [PubMed] [Google Scholar]
- Vawter MP, Evans S, Choudary P, Tomita H, Meador-Woodruff J, Molnar M, Li J, Lopez JF, Myers R, Cox D, Watson SJ, Akil H, Jones EG, Bunney WE. Gender-specific gene expression in post-mortem human brain: localization to sex chromosomes. Neuropsychopharmacology. 2004;29:373–384. doi: 10.1038/sj.npp.1300337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veney SL, Wade J. Steroid receptors in the adult zebra finch syrinx: a sex difference in androgen receptor mRNA, minimal expression of estrogen receptor α and aromatase. Gen. Comp. Endocrinol. 2004;136:192–199. doi: 10.1016/j.ygcen.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Veney SL, Wade J. Post-hatching syrinx development in the zebra finch: an analysis of androgen receptor, aromatase, estrogen receptor α and estrogen receptor β mRNAs. J. Comp. Physiol., A. 2005;191:97–104. doi: 10.1007/s00359-004-0577-5. [DOI] [PubMed] [Google Scholar]
- Wade J. Zebra finch sexual differentiation: the aromatization hypothesis revisited. Microsc. Res. Tech. 2001;54:354–363. doi: 10.1002/jemt.1148. [DOI] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. In: Zeigler HP, Marler P, editors. Behavioral Neurobiology of Birdsong. vol. 1016. New York Academy of Sciences; New York: 2004. pp. 540–559. [DOI] [PubMed] [Google Scholar]
- Wade J, Tang YP, Peabody CP, Tempelman RJ. Enhanced gene expression in the forebrain of hatchling and juvenile male zebra finches. J. Neurobiol. 2005;64:224–248. doi: 10.1002/neu.20141. [DOI] [PubMed] [Google Scholar]