Abstract
Background/purpose
In a recent issue of Experimental Dermatology (2009;18:654 – 655) Schefzyk and colleagues concluded that multi-antibody eosinophil isolation (Miltenyi) should be abandoned, as differential purity was minimal, and eosinophils underwent accelerated apoptosis when compared to those isolated with traditional anti-CD16 microbeads. Our intent was to investigate the universality of these findings.
Methods
We isolated eosinophils from normal donor granulocyte packs using the two methods, and evaluated purity, viability, and annexin-V/propidium-iodide staining.
Results
Purity was substantially greater when multi-antibody isolation was used for eosinophil isolation from granulocyte packs (98% vs. 69%). No differential survival was detected when eosinophils were maintained in culture with or without interleukin-5.
Conclusions
Multi-antibody eosinophil isolation represents a substantial advantage over anti-CD-16 microbeads when isolating large numbers of eosinophils from concentrated leukocyte preparations. No differential survival was observed. While appropriate consideration of methods is always crucial, multi-antibody eosinophil isolation should not be abandoned completely.
Keywords: eosinophil, interleukin-5, microbeads, CD16, apoptosis
Background
Eosinophils represent a minor population of leukocytes in the peripheral blood, typically 2 – 5% of the total leukocyte count under homeostatic conditions. Prior to the introduction of antibody-based selection profiles, eosinophils were isolated by Percoll gradients, a method that was difficult, time-consuming and yielded erratic results. In the 1990s, Miltenyi Biotec introduced a selection method involving anti-CD16 antibody-conjugated microbeads, which permitted the magnetic separation of untouched (CD16-negative) eosinophils from the CD16-positive neutrophils that co-migrate in Percoll gradient centrifugation; this represented an enormous advance in speed, simplicity and in final eosinophil purity. Interestingly, several groups reported that eosinophils isolated with anti-CD16 microbeads behaved differently in in vitro assays; compared to those isolated solely by Percoll gradient separation, anti-CD16 microbead-isolated eosinophils were unable to respond effectively to lipid chemoattracts or to interleukin-8 [1, 2], and appeared to be constitutively activated, over-producing leukotriene C4 and superoxide anion [3].
More recently, an expanded kit was released by Miltenyi which included multiple biotinylated antibodies (anti-CD2, CD14, CD16, CD19, CD56, CD123 and CD235a) followed by anti-biotin-conjugated microbeads, with the intent to improve eosinophil purity by antibody-based removal of peripheral blood mononuclear cells, rather than relying on the physical separation provided by the Ficoll/Hypaque gradient alone. However, in a recent manuscript, Schefzyk and colleagues [4] reported significant differences between eosinophils isolated using the multi-antibody isolation kit and those isolated using the original anti-CD16 microbeads. The authors concluded that the multi-antibody purification kit should be abandoned as the method yielded minimal increases in purity and was associated with accelerated eosinophil apoptosis.
Question addressed
As we use the multi-antibody isolation kit routinely, we asked, are the aforementioned findings universal or are they specific to the unique experimental conditions used in these authors’ experiments? The study performed by Schefzyk and colleagues [4] focused on purity and differential viability of eosinophils from atopic and normal blood donors; we focused on another use, specifically, isolation of large numbers eosinophils from normal donor granulocyte packs. Given the degree of leukocyte density in these packs, attaining a high degree of eosinophil purity represents a greater challenge.
Experimental Design
Granulocytes (50 mL samples) were collected from self-reported normal, non-cytokine-stimulated donors via a CS 3000 cell separator (NIH Clinical Center study number 99-CC-0168) and were isolated further via centrifugation over Cappel LSM lymphocyte separation medium (MP Biomedicals, LLC). The high-density granulocyte-red blood cell fraction was collected, and hypotonic lysis (distilled water) was performed to remove red blood cells. Eosinophils were isolated either by using the Miltenyi CD16 MicroBeads Kit (catalog number #130-045-701) or the Miltenyi Eosinophil Isolation Kit (#130-092-010) following the manufacturer’s instructions. Viability at isolation and at all time points thereafter was determined via trypan blue dye exclusion. Cytospin preparations stained with modified Giemsa were used to determine cell differentials. Isolated eosinophils were cultured at 106/mL in RPMI with 20% heat-inactivated fetal calf serum, 2 mM glutamine, 25 mM HEPES, 50 uM beta-mercaptoethanol, 100 U/mL penicillin-streptomycin, 1X non-essential amino acids (Invitrogen), 100 mM sodium pyruvate, with or without 25 ng/mL interleukin-5 (R&D Systems). Flow cytometric analsysis was performed on a BD LSR II analyzer using a FITC annexin V apoptosis detection kit I (BD Pharmingen, cat. no. 556547). Multi-antibody purified eosinophils sustained with 25 ng/mL IL-5 were subjected to experiments that utilized the degranulation assay described by Adamko and colleagues [5].
Results
Schefzyk and colleagues [4] reported only minimal differences in eosinophil purity when using peripheral blood from atopic donors as source material. Although we were also able to isolate eosinophils at high purity (~97%) using anti-CD16 microbead isolation with peripheral (whole) blood as source material [6], we found pronounced differences in purity when using each of the two kits to isolate eosinophils from normal donor granulocyte packs. While ultimate yield of eosinophils per patient sample is high (2 × 108 to 109 per 50 mL granulocyte pack sample), the purity obtained with anti-CD16 microbeads alone is substantially lower, typically only ~70%, with contaminating cells, monocytes and neutrophils, vs. 97 – 99% purity attained using the multi-antibody based isolation method [Figure 1].
Figure 1.
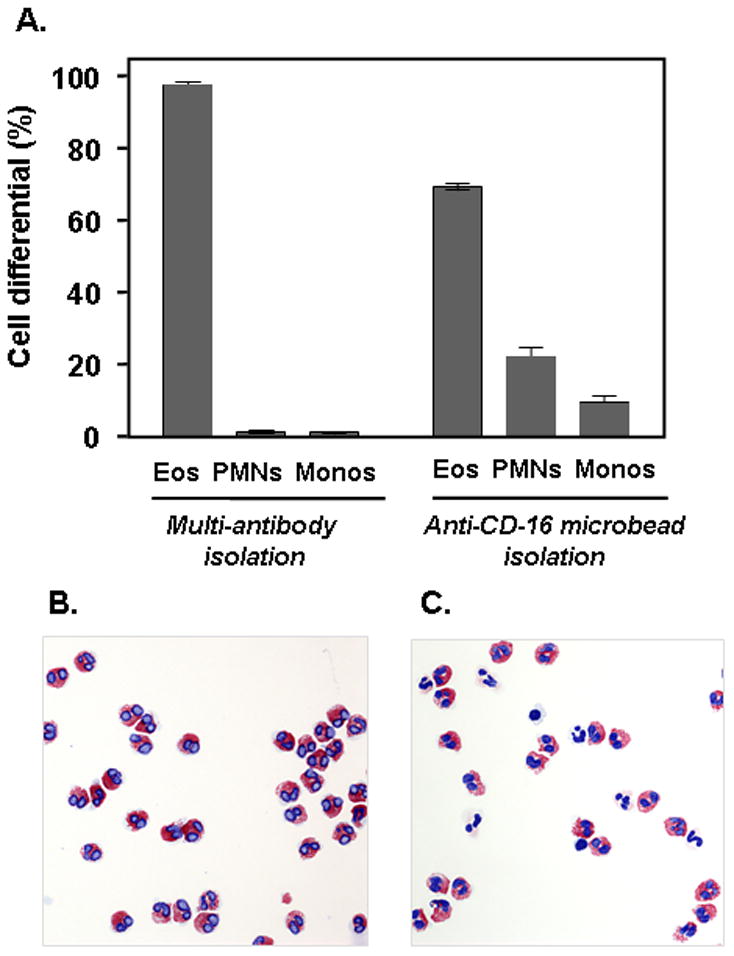
(A) Leukocyte differentials after isolation of eosinophils using the Milltenyi multi-antibody method compared to that obtained after isolation using the original anti-CD16 microbeads alone. Eos, eosinophils; PMNs, polymorphonuclear leukocytes, or neutrophils; Monos, monocytes. Differentials shown are based on 500 leukocytes counted for each of two separate isolations for each method; representative experiment of n = 3. (B) and (C) are cytospin preparations of cells from typical multi-antibody and anti-CD16 microbead isolations, respectively (modified Giemsa stain, original magnification, 20X).
Furthermore, Schefzyk and colleagues [4] found that survival of eosinophils in a basic culture without cytokine support was dependent on the method of isolation. In our hands, there was no differential survival of eosinophils from self-reported normal donors over 7 days in culture either sustained with IL-5 [Figure 2A] or devoid of cytokine support [Figure 2B]. Normal eosinophils isolated via multi-antibody and anti-CD16 isolation methods both undergo accelerated apoptosis in the absence of cytokine support compared to parallel cultures sustained with IL-5, measured here as propidium iodide negative/annexin V-positive staining after 24 h in culture [Figure 2C]. There was no evidence that the multi-antibody purification method accelerated this process further under either set of culture conditions.
Figure 2.
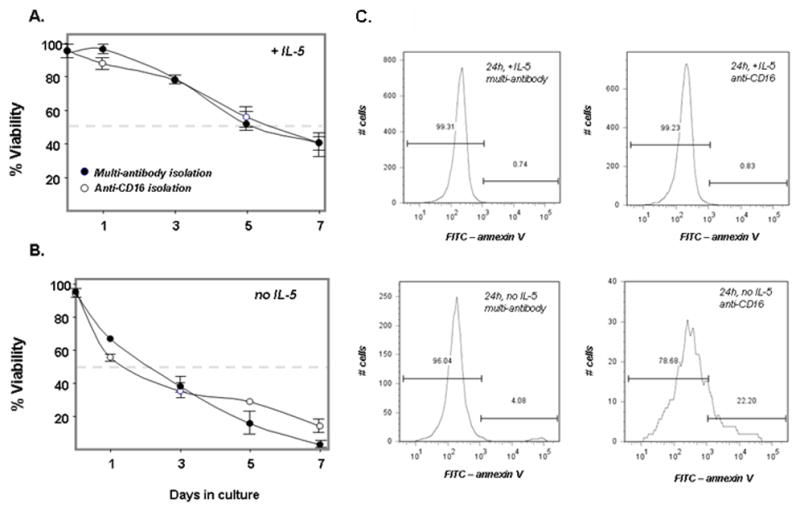
Eosinophil viability (%) measured by trypan blue exclusion (A) sustained by 25 ng/mL IL-5 and (B) in the absence of IL-5; filled circles, multi-antibody isolation method; open circles, anti-CD16 isolation method. Horizontal broken line indicates 50% survival; representative experiment of n = 2; (C) Apoptosis documented as propidium iodide-negative/annexin V-positive staining of eosinophils isolated using the multi-antibody or anti-CD-16 microbead isolation, cultured for 24 hours either with or without 25 ng/mL IL-5, as indicated; representative experiment of n = 2.
Discussion
We cannot state with any clarity that our findings disprove or contradict those of Schefzyk and colleagues [4]; rather, we find their conclusion – that the multi-antibody based method should not be used to isolate eosinophils designated for functional studies – to be much too broad and far-reaching. While these authors demonstrated that eosinophils from atopic and normal blood donors isolated via the multi-antibody method underwent more rapid loss of viability than those isolated by the anti-CD16 microbeads, we determined that eosinophils from normal donor granulocyte packs were sustained with equivalent viability over a full seven day period, both with cytokine support and without. Furthermore, in our hands, using the higher density source of enriched granulocytes, the differential purity obtained was more pronounced, providing a greater impetus to utilize the multi-antibody purification method. We have used multi-antibody purified eosinophils sustained in culture with IL-5 for our studies of eosinophil-virus interactions [[7]; Supplemental Figure 1], which are experiments that require prolonged eosinophil viability in culture.
Conclusions
In conclusion, it is certainly important to examine all changes in purification methods and to proceed with caution when considering even seemingly minor modifications. However, as we have shown, there are advantages inherent in the multi-antibody eosinophil purification method for purification of eosinophils from high density leukocyte packs. Furthermore, the differential impact on viability reported by Schefzyk and colleagues [4] is apparently not universal, as it was not detected in cultures of eosinophils from normal donors.
Supplementary Material
Acknowledgments
The authors thank Ms. Cynthia Mathews of the NIH Clinical Center Blood Bank for her assistance and coordination in obtaining the granulocyte packs and Mr. Rick Dreyfuss of Medical Arts for his assistance with the photographic imaging. This study was supported by funding from NIAID Division of Intramural Research.
References
- 1.Casale TB, Erger RA, Rozell MD. Eosinophils isolated by magnetic cell sorting respond poorly to lipid chemoattractants. Ann Allergy Asthma Immunol. 1999;83:127 – 131. doi: 10.1016/S1081-1206(10)62623-3. [DOI] [PubMed] [Google Scholar]
- 2.Rozell MD, Erger RA, Casale TB. Isolation technique alters eosinophil migration response to IL-8. J Immunol Methods. 1996;197:97 – 107. doi: 10.1016/0022-1759(96)00132-9. [DOI] [PubMed] [Google Scholar]
- 3.Sedgwick JB, Shikama Y, Nagata M, Brener K, Busse WW. Effect of isolation protocol on eosinophil function: Percoll gradients versus immunomagnetic beads. J Immunol Methods. 1996;198:15 – 24. doi: 10.1016/0022-1759(96)00139-1. [DOI] [PubMed] [Google Scholar]
- 4.Schefzyk M, Bruder M, Schmiedl A, Stephan M, Kapp A, Wedi B, Raap U. Eosinophil granulocytes: functional differences of a new isolation kit compared to the isolation with anti-CD16-conjugated MicroBeads. Exp Dermatol. 2009;18:654 – 655. doi: 10.1111/j.1600-0625.2008.00824.x. [DOI] [PubMed] [Google Scholar]
- 5.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291:101 – 108. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Domachowske JB, Rosenberg HF. Eosinophils inhibit retroviral transduction of human target cells by a ribonuclease-dependent mechanism. J Leukoc Biol. 1997;62:363–368. doi: 10.1002/jlb.62.3.363. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg HF, Dyer KD, Domachowske JB. Respiratory viruses and eosinophils: exploring the connections. Antiviral Res. 2009;83:1–9. doi: 10.1016/j.antiviral.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


