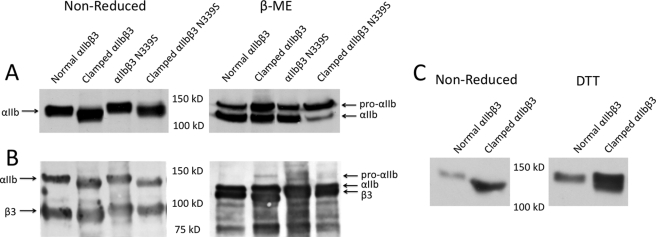
FIGURE 2.
Clamped αIIb migrates more rapidly under nonreducing conditions in SDS-PAGE than normal αIIb; reducing disulfide bonds with β-mercaptoethanol eliminates the difference in migration. A, total cell analysis. Lysates of cells expressing normal and clamped αIIb were heated to 100 °C in the presence or absence of 10% β-mercaptoethanol (β-ME) before loading on a 7% polyacrylamide gel. After protein transfer to polyvinylidene fluoride membranes, αIIb was detected by the anti-αIIb mAb PMI-1. B, surface receptor analysis. The cells were surface-labeled with biotin and then lysed and immunoprecipitated with a mAb to αIIbβ3. The proteins were separated by SDS-PAGE, and then biotin-labeled proteins were identified with avidin-horseradish peroxidase. C, nonreduced SDS-PAGE analysis of normal and clamped αIIb before and after treating cells with 5 mm DTT. Note that the increased migration of clamped αIIb is largely, but not completely, reversed by 5 mm DTT.