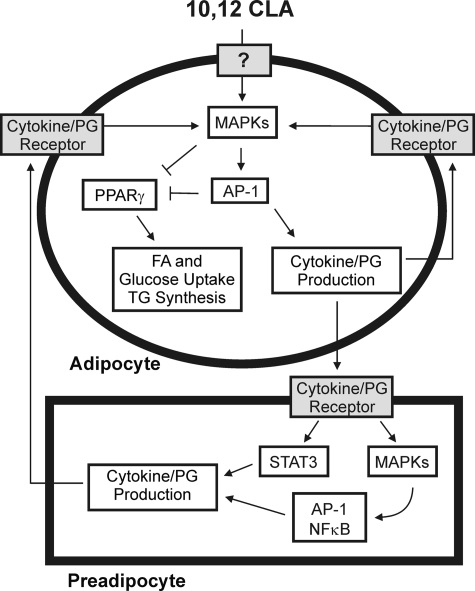
FIGURE 10.
Working model. Treating primary cultures of newly differentiated human adipocytes with 10,12-CLA increases the phosphorylation of MAPK (i.e. ERK 1/2, JNK, and p38) and the activation of the transcription factor AP-1 (i.e. c-Jun, c-Fos, ATF2, and ATF3), which leads to the production of inflammatory cytokines and PGs (i.e. PGE2 and PGF2) through up-regulation of inflammatory genes (i.e. COX-2, IL-1β, IL-8, and ATF3). These inflammatory signals subsequently activate preadipocytes leading to inflammatory cytokine secretion from preadipocytes, thus continuing the inflammatory cycle. Furthermore, activation of STAT3, MAPK, and AP-1 by 10,12-CLA antagonizes PPARγ and associated target genes, ultimately leading to delipidation through decreased glucose and fatty acid uptake and decreased TG content in adipocytes.