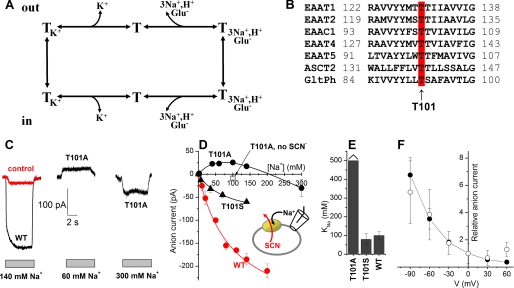
FIGURE 1.
Mutation of Thr101 to alanine interferes with the ability of Na+ to induce anion current. A, illustration of the transport cycle. B, multiple sequence alignment of EAATs and GltPh shows that Thr101 (red, arrow) is conserved within the glutamate transporter family. C, typical leak anion currents induced by Na+ application to EAAC1WT in the absence of external substrate (left panel, black), nontransfected control cells (left panel, red), and EAAC1T101A (middle and right panels). The gray bars indicate the length of Na+ application. The extracellular solution contained MES as the anion and Na+/NMG+. The intracellular solution contained 140 mm SCN− and 10 mm glutamate (see inset in C). D, [Na+] dependence of specific leak anion currents (red circles, WT; black circles, T101A; black triangles, T101S, control responses were subtracted; open square, control response in the absence of SCN−). The lines represent fits to the Michaelis-Menten equation (WT, T101S) or a sum of two Michaelis-Menten equations (T101A). E, KNa+ values determined from the fits. F, voltage dependence of the 60 mm Na+-induced apparent outward current (T101A, open circles) indicates that it is mediated by inhibition of a tonic inward anion conductance. For comparison, inhibition of the tonic leak anion conductance of EAAC1WT by TBOA as a function of the voltage is shown as the solid circles.