FIGURE 4.
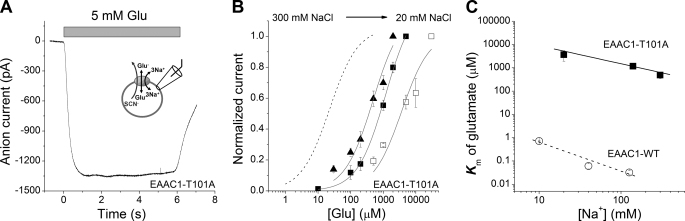
Increased occupancy of the Na+ binding site(s) increases the glutamate affinity of EAAC1. A, typical anion current in response to 5 mm glutamate applied to EAAC1T101A in the exchange mode (see inset). B, normalized glutamate dose-response curves as a function of extracellular [Na+] (300 mm (▴), 140 mm (■), and 20 mm (□)) in exchange mode. The dotted line represents the wild type curve at 140 mm Na+. The currents were normalized to the response at 30 mm glutamate (20 mm Na+), 5 mm glutamate (140 mm Na+), and 2 mm glutamate (300 mm Na+). The extracellular solution contained MES as the anion and the indicated Na+ concentration supplemented with NMG+ to 300 mm. The composition of the internal solution was 140 mm NaSCN and 10 mm glutamate, and the transmembrane potential was 0 mV. C, [Na+] dependence of the Km of EAAC1WT (open circles) and EAAC1T101A (closed squares) for glutamate.