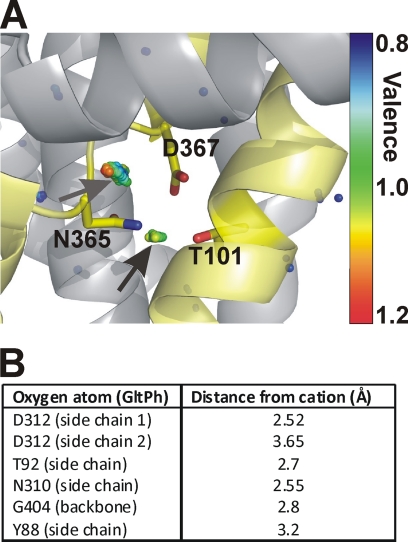
FIGURE 9.
Valence mapping of the GltPh structure predicts a potential cation binding site with the side chains of Asp367 and Thr101 as ligands. A, valence map for K+ (colored dots) in overlay with the GltPh structure. The dots illustrate points in three-dimensional space that were mapped to a valence of 0.8 (blue) to 1.2 (red) (the valence color code is shown on the right side of the figure). An optimal valence of 1 is color-coded green. Thus, green dots indicate a suitable binding site for K+, as indicated by the arrows. Residues Thr92, Asn310, and Asp312 of GltPh (Thr101, Asn365, and Asp367 in EAAC1) are highlighted as sticks. B, distances of the coordinating oxygen atom to the center of the cation used for valence calculation.