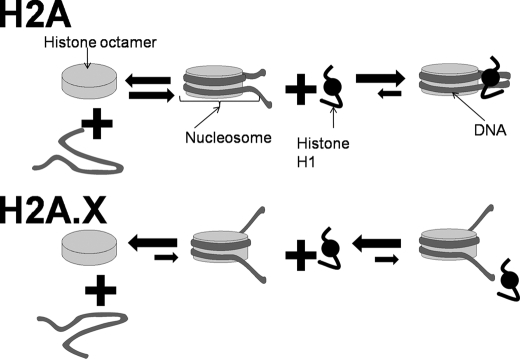
FIGURE 7.
Model proposed to account for the structural implications of histone H2A replacement by H2A.X and its C-terminal phosphorylation. As indicated in the left-hand side of this figure, isolated nucleosomes in solution exhibit a reversible dissociation into its constitutive histone and DNA components that depends on temperature, concentration, and ionic strength (51). Histone H2A.X decreases the stability of the nucleosome, displacing this equilibrium toward the dissociated state (Fig. 5). In addition and as shown in the lower part of this figure, histone H2A.X alters the nucleosome conformation (Fig. 6C) in a way that impairs the binding of linker histones (Fig. 6, A and B).