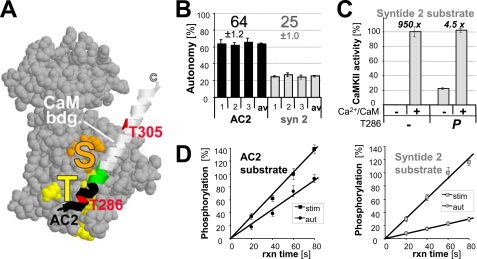
FIGURE 1.
CaMKII autonomy is substrate-dependent. A, CaMKII kinase domain (19) with the autoregulatory α-helix shown as a ribbon, containing the CaM binding site (white), Thr-286, and Thr-305 (red). The substrate binding S-site (orange) and the Thr-286 region binding T-site (yellow) are marked. AC2 is derived from the region shown in black. B, CaMKII (2.5 nm) autonomy toward the substrate peptides AC2 and syntide2 (75 μm) differed significantly from each other on three experimental days. Autonomy reflects the activity of Thr-286-autophosphorylated CaMKII after chelating Ca2+ with EGTA, relative to the activity of naïve CaMKII maximally stimulated by Ca2+/CaM (2 μm). C, Ca2+/CaM stimulated naïve CaMKII 950-fold, but still stimulated autonomous CaMKII 4.5-fold, at least for syntide2. D, measurement of stimulated (stim; Ca2+/CaM) and autonomous (aut; EGTA) CaMKII activity was in the linear range, as ascertained by stopping the reactions at different time points. Stimulated phosphorylation after 60 s was set as 100%. Error bars indicate S.E. in all panels.