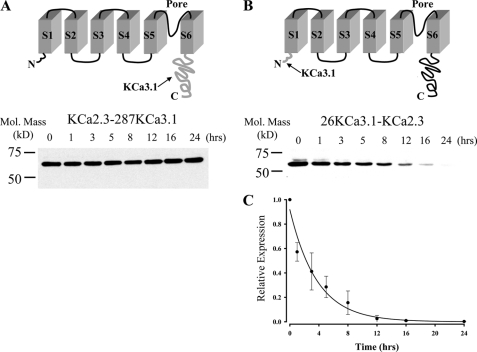
FIGURE 10.
N terminus of KCa2.3 is deterministic for its long half-life. A, top panel illustrates a model in which the C terminus of KCa3.1 (amino acids Arg287–Lys427; shown in gray at arrow) is appended onto S6 of KCa2.3 (KCa2.3–287KCa3.1). Bottom panel shows a representative IB for the degradation of this chimera expressed in HEK cells in the presence of cycloheximide (400 μg/ml) for the times indicated. No apparent degradation was observed over a 24-h period. 20 μg of total protein was loaded per lane. Similar results were observed in three separate experiments. B, top panel illustrates a model in which the N terminus of KCa3.1 (amino acids Met1–Ala26; shown in gray at arrow) is appended on to S1 of KCa2.3 (26KCa3.1-KCa2.3). Bottom panel shows a representative IB for the degradation of this chimera expressed in HEK cells in the presence of cycloheximide (400 μg/ml) for the times indicated. 20 μg of total protein was loaded per lane. C, data from three separate experiments on 26KCa3.1-KCa2.3 (B) were digitized and plotted as a fraction of protein at time 0. The data were fitted to an exponential decay function with a time constant for degradation of 3.7 ± 0.3 h (n = 3).