FIGURE 4.
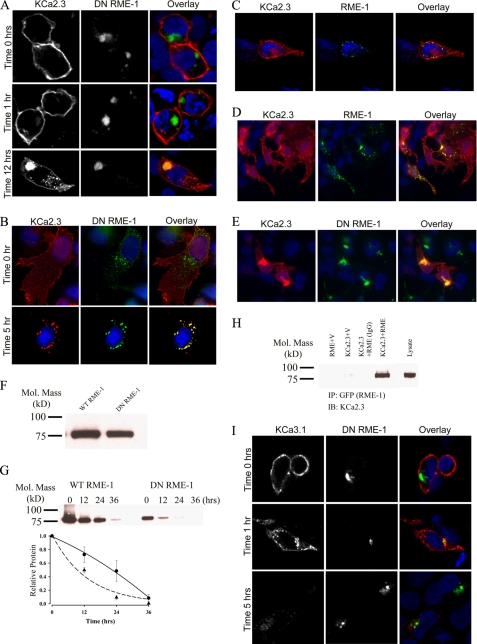
KCa2.3 enters RME-1-positive endosomes in HEK and HMEC-1 cells, whereas KCa3.1 does not. A, BLAP-tagged KCa2.3 was co-expressed with GFP-tagged DN RME-1 in HEK cells. KCa2.3 was labeled with streptavidin-Alexa555 and localization evaluated after 0, 1, and 12 h at 37 °C. KCa2.3 is localized to the plasma membrane at 0 h (upper panels), and after 1 h (middle panels) very little channel is localized to intracellular vesicles. However, after 12 h (lower panels), KCa2.3 has accumulated in the RME-1-expressing recycling compartment. B, BLAP-tagged KCa2.3 was co-expressed with GFP-tagged DN RME-1 in HMEC-1 cells, labeled as above, and localization evaluated after 0 (top panels) or 5 (bottom panels) h at 37 °C. At time 0, all of KCa2.3 is localized to the plasma membrane, whereas there is nearly complete co-localization of KCa2.3 with the RME-1-positive recycling compartment following endocytosis. C, BLAP-tagged KCa2.3 was co-expressed with GFP-tagged RME-1 in HEK cells. KCa2.3 was labeled as above, and localization was evaluated after 3 h at 37 °C. D and E, KCa2.3 (no BLAP tag) was co-expressed with either wild type (D) or DN (E) GFP-tagged RME-1 and steady-state localization assessed by IF. F, immunoblot of total lysate following co-expression of KCa2.3 with WT RME-1 (1st lane) or DN RME-1 (2nd lane). DN RME-1 reduced KCa2.3 expression an average of 30 ± 3% (n = 3). G, KCa2.3 was co-expressed with either WT (left panel) or DN (right panel) RME-1, and cell surface channel was biotinylated. At the indicated times, cells were lysed, subjected to streptavidin pull-down, and blotted for KCa2.3. At time 0, DN RME-1 reduced plasma membrane KCa2.3 expression an average of 62 ± 3.5% (p < 0.05, n = 4). The bands were quantified by densitometry, normalized, and plotted as a function of time (bottom panel) for DN (dashed line, triangles) and WT (solid line, circles) RME-1. H, co-immunoprecipitation of KCa2.3 with GFP-tagged RME-1 was carried out in HEK cells. RME-1 was immunoprecipitated via its GFP tag, and the subsequent blot was probed for KCa2.3. Cells expressed either RME-1 plus vector (1st lane), KCa2.3 plus vector (2nd lane), or KCa2.3 plus GFP-RME-1 (3rd and 4th lanes). The V5 Ab was used as an IgG control in the 3rd lane. 10 μg of total protein was loaded in the lysate lane to confirm the identity of KCa2.3. I, BLAP-tagged KCa3.1 was co-expressed with GFP-tagged DN RME-1 in HEK cells. KCa3.1 was labeled with streptavidin-Alexa555, and localization was evaluated after 0, 1, and 5 h at 37 °C. In contrast to KCa2.3, KCa3.1 does not enter the RME-1-positive compartment and is rapidly degraded, similar to what was observed in the absence of DN RME-1. In all experiments, co-localization is shown in the overlay image as yellow. Nuclei are labeled with DAPI (blue). Images are either single confocal sections (A and I) or projection images (B–E).