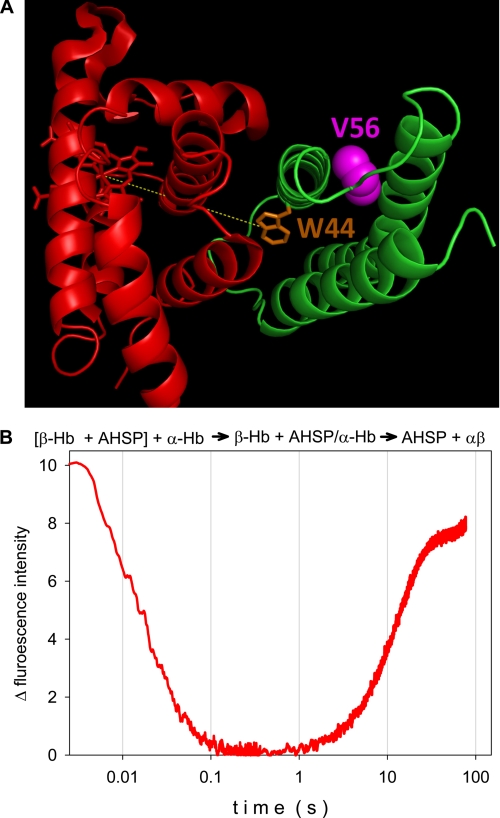
FIGURE 1.
A, three-dimensional view of the complex of AHSP (green) and α-Hb (red). The molecular structure shows the position of the AHSP tryptophan residue Trp44 (orange) and the valine (violet) that is mutated (V56G) in the clinical case. The image was obtained from the crystallographic structure of the AHSP·α-Hb complex (Protein Data Bank code 3ia3) using PyMOL software. B, composite kinetics of the AHSP association with α chains and subsequent replacement of AHSP by β chains at 37 °C, pH 7. Mixing ratios were 1:1 in each case at 10 μm final protein concentration. The β chain and AHSP were premixed and loaded in one of the stopped-flow syringes, for mixing with the α chains: [β-Hb + AHSP] + α-Hb → β-Hb + AHSP·α-Hb → AHSP + αβ. The fluorescence of the single tryptophan (Trp44) residue of AHSP serves as a probe for the binding to α chains.