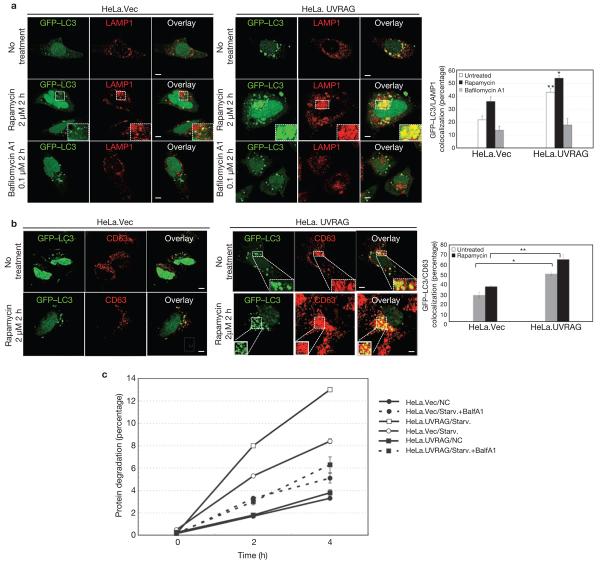
Figure 4. Autophagosome maturation in UVRAG-expressing HeLa cells.
(a) UVRAG enhances the colocalization efficiency of GFP–LC3 with LAMP1. HeLa.Vec and HeLa.UVRAG cells transfected with GFP–LC3 were either untreated or treated with rapamycin (2 μM) in the absence or presence of bafilomycin A1 (0.1 μM) and stained for LAMP1. Insets highlight the colocalization. The percentage of LAMP1-positive autophagosomes was calculated in each setting (data are mean ± s.e.m., n = 200; 5 independent experiments, **P < 0.01; *P < 0.05). (b) UVRAG enhances the acquisition of CD63 by autophagosomes. The GFP–LC3-transfected HeLa.Vec and HeLa.UVRAG cells were treated as described in a and stained for CD63 and the percentage of CD63-positive autophagosomes was calculated (data are mean ± s.e.m., n = 200, **P < 0.01; *P < 0.05). (c) UVRAG promotes autophagic degradation of long-lived proteins. HeLa.Vec and HeLa.UVRAG cells were incubated for 16 h with l-3H-Leu (1 μCi ml−1). The degradation of long-lived proteins was measured at the indicated time points in complete medium (NC), EBSS alone (Starv.), or EBSS + 0.1 μM bafilomycin A1 (Starv.+BalfA1). Results (data are mean ± s.e.m. of triplicates) are representative of 2 independent experiments. Scale bars, 5 μm.