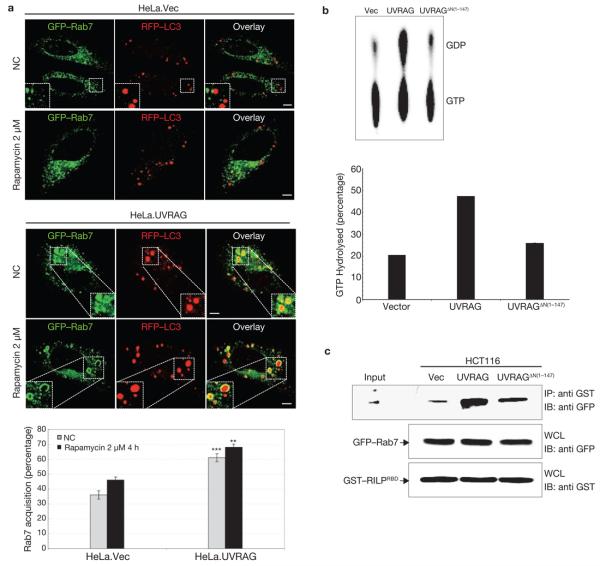
Figure 6. Rab7 acquisition and activation by UVRAG.
(a) UVRAG expression promotes the recruitment of Rab7 GTPase to autophagosomes. HeLa. Vec and HeLa.UVRAG cells transfected with GFP–Rab7 and RFP–LC3 were maintained either under normal conditions (NC) or treated with rapamycin, followed by confocal microscopy. Insets highlight the Rab7 acquisition of autophagosomes. The percentage of Rab7+-autophagosomes was quantified (right panel; data are mean ± s.e.m., n = 60, ***P < 0.001; **P < 0.01). Scale bars, 5 μm. (b) Rab7 GTPase activity is increased with UVRAG expression. At 48 h post-transfection with GFP–Rab7 together with vector, UVRAG or UVRAGΔN(1–147) vector, 293T WCLs were used for immunoprecipitation with anti-GFP, followed by the Rab7 GTPase activity assay. Left panel: Autoradiographs of the GTP hydrolysis products analysed by TLC. Right panel: Quantification of the percentage of GTP hydrolyzed by Rab7 (data are mean n = 2 independent experiments). (c) Increased Rab7–RILP interaction UVRAG is expressed. HCT116.vector, HCT116.UVRAG and HCT116.UVRAGΔN(1–147) cells were transfected with GFP–Rab7 together with the GST-tagged Rab7 binding domain of RILP (GST–RILPRBD, residues 243–318). WCLs were used for GST pulldown, followed by immunoblotting with an anti-GFP antibody. The raw data of the immunoblots are shown in Supplementary Information, Fig. S6.