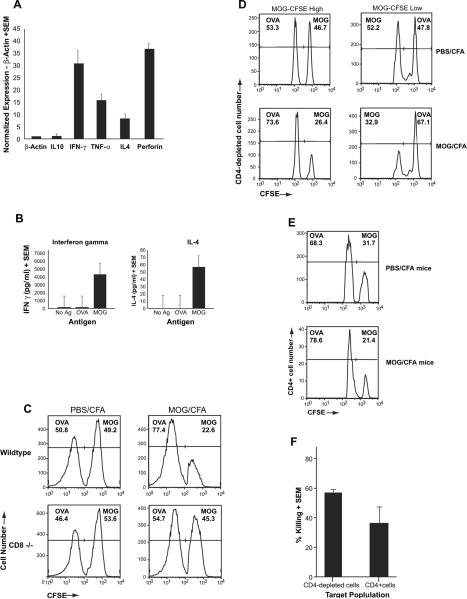
Fig. 4.
Cytokine expression and cytotoxic function of MOG-specific CD8+ T cells. (A) Total RNA was extracted from in vitro stimulated MOG35–55-specific CD8+ T cells. Relative expression of IL-10, IFN-γ, TNF-α, IL-4 and Perforin were assessed using a quantitative PCR (qPCR) assay and normalize using the housekeeping gene β-actin. (B) CD8+ T cells were purified by negative selection from splenocytes of MOG35–55-immunized mice at day 20 post-immunization. These cells were cultured with irradiated APC and stimulated with the indicated antigen. Supernatants were collected at 72 h of culture and assayed for IFN-γ or IL-4 by ELISA. (C) Splenocytes from naïve B6 mice were harvested and stained for different levels of CFSE: CFSE-high and CFSE-low. Following staining, the cells were incubated with Con A (10 μg/ml) in the presence of either MOG35–55 (20 μg/ml) or OVA323–339 (20 μg/ml) for 18–20 h. Cells from each group were then counted and mixed at a 1:1 ratio and injected into either wildtype or CD8(−/−) mice that had been immunized 25 days prior with MOG35–55/CFA or PBS/CFA. A total of 2 days after injection of target cells, splenocytes harvested and analyzed by flow cytometry. This figure is a representative of three experiments and shows disappearance of the MOG-loaded target cells only in wildtype MOG-immunized mice. (D & E) Target cells were split into either CD4-depleted APC (D) or CD4+ T cells (E) after loading with either control antigen (OVA323–339) or MOG35–55. These cells were injected into PBS/CFA- or MOG/CFA-immunized mice at day 20 post-immunization and in vivo killing was evaluated and normalized to the ratios of the injected cells in the CFA-immunized mice. (E) Cumulative graphic representation of percent killing determined by comparing ratio of CFSE-low and CFSE-high cells in MOG-immunized mice to that of CFA control mice.