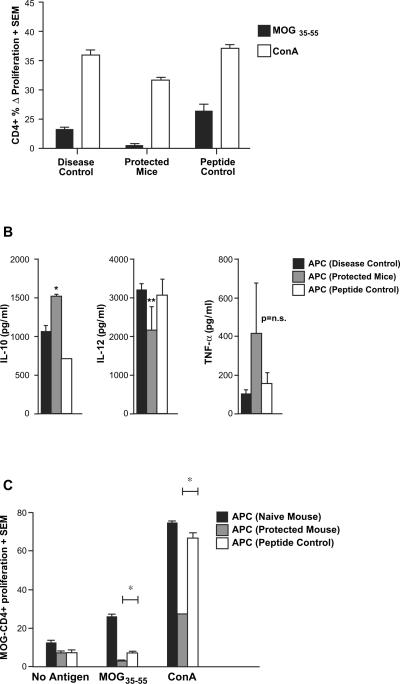
Fig. 5.
MOG35–55-specific CD8+ T cells modulate MOG-specific CD4+ T cell responses as well as APC function. In vitro-activated MOG- (protected mice) or OVA-specific (peptide control) CD8+ T cells mice were injected into naïve B6 mice, followed by immunization with MOG35–55/CFA (similar to experiments in Fig. 3B). A third group of mice only received MOG35–55/CFA immunization without any transfer of cells (disease control). At day 15 post-immunization, lymph node cells as well as splenocytes were harvested. (A) MOG-specific CD4+ T cell responses were quantified by CFSE-based proliferation assays and are depicted as Δ% proliferating fraction (“no antigen” background subtracted), showing significant inhibition (p < 0.05) of these responses in mice receiving MOG-specific CD8+ T cells. (B) T-depleted antigen presenting cells (APC) from the protected mice [APC (protected mice)], OVA control mice [APC (peptide control)] and no transfer control [APC (disease control)] were stimulated with LPS. Supernatants from these cultures were extracted at various time points and tested by ELISA for the indicated cytokines. APC from protected mice showed significantly higher IL-10 secretion (*p < 0.05) and a trend toward lower IL-12 secretion (p = 0.07). TNF-α differences were not statistically significant. (C) APC from the three groups of mice were also used to stimulate purified CD4+ cells from MOG35–55-immunized mice (used as responders). The magnitudes of proliferation from a CFSE-based assay are depicted. All results are representative of two replicate experiments.