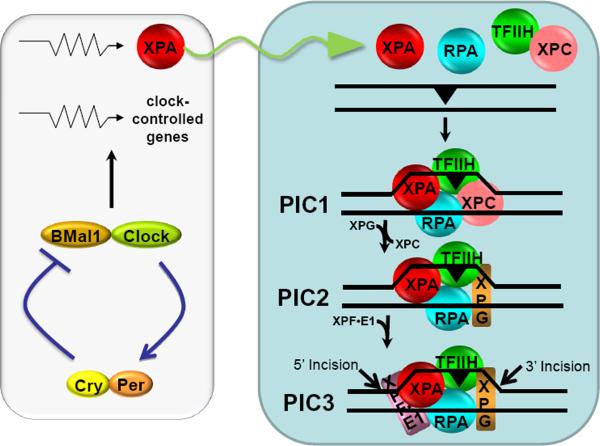
Figure 3.
Model for nucleotide excision repair in humans and its regulation by the circadian clock. Excision of DNA damage is accomplished by sequential and partially overlapping functions of 6 core repair factors. Damage is recognized by cooperative activities of RPA, XPA, and XPC, followed by recruitment of TFIIH by XPC and XPA. The DNA around the damage site is unwound by the helicase activity of TFIIH to form a stable pre-incision complex 1 (PIC1) which recruits XPG, and XPC is displaced from the complex to form PIC2. Then XPF-ERCC1 is recruited to form PIC3. Within PIC3, XPG makes the 3′ incision 6±3 phosphodiester bonds 3′ and XPF makes the 5′ incision 20±5 phosphodiester bonds 5′ to the damage. The excised 24-32 nucleotide-long oligomer carrying the damage is released and the resulting gap is filled by DNA polymerases and ligated. The XPA protein which plays an essential role in damage recognition and is a rate-limiting factor is regulated by the clock, and as a consequence the daily oscillation of XPA (sinusoidal arrow) causes the entire excision repair activity to exhibit a daily rhythm, increasing during the day and decreasing during the night. Modified from [9, 66].