Abstract
Enzyme-linked immunosorbent assay (ELISA) and Western blotting are common techniques used to detect and quantify proteins in Staphylococcus aureus culture supernatants, such as Panton-Valentine leukocidin (PVL). However, protein A (Spa) secreted by most S. aureus strains may interfere with these assays by binding to the capturing and detecting antibodies. Here, we have shown that the addition of diethylpyrocarbonate (DEPC) inhibits the binding of Spa to rabbit anti-PVL used as the capturing antibody in ELISA. In Western blotting, the presence of DEPC prevented the binding of detecting antibody to Spa. These modified ELISA and Western blot techniques should prove useful for detecting and quantifying proteins in S. aureus cultures supernatants.
Keywords: Staphylococcus aureus, protein A, Panton-Valentine leukocidin, Diethylpyrocarbonate, ELISA, Western Blot, Quantification, Detection
1. Introduction
A number of immunological techniques including ELISA and Western blot have been described for detecting and quantifying staphylococcal proteins. The 42 kD Staphylococcus aureus protein A (Spa) secreted by 99% of S. aureus isolates (Goding, 1978) interferes with the ELISA by binding to the Fc region of most mammalian IgGs used as the capturing antibodies in these assays. To prevent protein interference several techniques have been employed, but most have problems of their own. F(ab′)2 fragments have been used as capturing reagents, but Spa binds to Fab's containing VH3 (Sasso et al., 1991; Ladhani et al., 2001). Affinity chromatography or addition and centrifugation of porcine IgG coupled to insoluble matrix has been used to remove protein A from culture supernatants (Berdal et al., 1981; Fey and Burkhard, 1981). Detecting rabbit antibodies have been biotinylated so that the binding site of Spa on the IgG molecule was masked (Hahn et al., 1986). Other methods have relied on the development of capturing IgG antibodies in rats (Rogemond et al., 1991) and sheep (Freed et al., 1982) that bind only weakly to Spa. In addition, chicken anti-protein A IgG has been used to sequester protein A, since chicken IgG does not bind protein A in the Fc region (Hoffman et al., 1996). These approaches developed to overcome the Spa interference require a considerable amount of time and additional expense.
Here, we describe the development of sandwich ELISA and Western blotting techniques to detect and estimate the amount of the LukS-PV component of Panton-Valentine leukocidin (PVL) secreted by community-associated methicillin resistant S. aureus (CA-MRSA). We show that the addition of diethylpyrocarbonate (DEPC) inhibits the interaction of protein A present in the culture supernatants containing PVL with the capturing antibody. This approach can be used to detect other proteins when interference with protein A is a problem.
2. Materials and Methods
2.1 Bacterial Preparation
Four clinical CA-MRSA isolates were used in this study: LAC (provided by F. DeLeo, NIH), SA-123, SA-109, and SA-112. LAC and SA-123 are pvl+ strains that produce different amounts of PVL proteins; SA-109 is a pvl- strain and SA-112 is pvl+ but produces very low levels of Spa. Bacteria were grown in YCP (yeast, casamino acid, pyruvate) medium for 18 hours at 37°C with constant shaking from glycerol stocks stored at -30°C. Bacterial densities were determined by spectrophotometry (O.D. 600 nm) then converted to estimated colony forming units (CFUs) using a standard curve. Supernatants were collected after centrifugation (3500 rpm) for 5 minutes at 4°C.
2.2 Construction of LukS-PV recombinant protein
PVL is a bicomponent toxin encoded by two genes, lukS-PV and lukF-PV (Kaneko and Kamio, 2004). The gene encoding LukS-PV was amplified by PCR from DNA extracted (DNeasy Tissue Kit, Qiagen) from a pvl+ S. aureus strain (49775, ATCC). The PCR products were ligated into pET151D/TOPO (Invitrogen) and the resultant plasmid was transformed into E. coli BL21 (Invitrogen) and grown in LB media with ampicillin at 37°C in the presence of 1 mM IPTG. Recombinant LukS-PV (rLukS-PV) protein was purified over a nickel-cadmium column and the His-6 tag was removed from the recombinant protein using AcTEV protease (Invitrogen).
2.3 Primary and secondary antibody preparation
Both rabbits and mice were immunized with 100 μg AcTEV-cleaved LukS-PV protein in emulsion with complete Freund's adjuvant on day 0, followed by booster immunizations in emulsion with incomplete Freund's adjuvant on days 7, 14 and 28. Antibody titers were determined by ELISA plates coated with rLukS-PV. Animals were terminally bled after satisfactory antibody titers (≥1:500,000) were reached. To rule out the possibility of cross-reactivity with other sequence related staphylococcal cytotoxins, reactivity to alpha toxin (Sigma) and native LukS-PV, LukF-PV, HlgA, HlgB and HlgC purified from culture supernatants as previously described (Prevost et al., 1995) was analyzed by SDS-PAGE and Western blotting using rabbit anti-LukS-PV as the detecting antibody (data not shown). Both rabbit and mouse antisera bound specifically to the immunizing protein only.
2.4 ELISA
Microtiter plates were coated (100 μL/well) with rabbit anti-LukS-PV diluted 1:500 in 50 mM carbonate buffer, pH 9.6 by incubating at 37°C for 2 hrs. Unbound sites were blocked with PBS containing 5% skim milk by incubating overnight at 4°C. Staphylococcal supernatants were then added to the rabbit antibody-coated plates (100 μL/well) then incubated at 37°C for 1 hour. After washing three times with PBS, the plates were incubated at 37°C with mouse anti-LukS-PV antibody diluted 1:500 in PBS containing 5% skim milk for 1 hour. After washing three times with PBS, the plates were incubated with goat anti-mouse IgG conjugated to alkaline phosphatase (Southern Biotech) at 1:500 dilution (100 μL/well) followed by the substrate disodium p-nitrophenyl phosphate solution (Sigma) at a concentration of 1 mg/mL in 1 M diethanolamine, 0.25 mM MgCl, pH 9.8. After 1 hour of development at 37°C, the absorbance was read at OD 405 nm. To block the binding of Spa to rabbit anti-LukS-PV, rabbit antibody coated plates were incubated with DEPC (Sigma) diluted in PBS, pH 6.0 from a 5 M stock solution. To detect if Spa was binding to rabbit anti-LukS-PV coated plates, chicken anti-protein A conjugated to horseradish peroxidase (HRP, Genscript, Piscataway, NJ), was used as the detecting antibody.
2.5 Western blot for LukS-PV
Bacterial supernatants were subjected to one-dimensional SDS-PAGE on 12% polyacrylamide gels for 1 hour at 80 mV. Proteins were transferred to 0.45 μm polyvinylidene fluoride membranes (Pierce) for 30 minutes at 25 mV. Membranes were then incubated with rabbit anti-LukS-PV (1:500) diluted in PBS containing 5% skim milk, pH 6.0 in the presence of DEPC for 20 minutes at room temperature. Membranes were then washed three times with PBS, pH 7.4 prior to incubation with an anti-rabbit IgG conjugated to HRP (Pierce) at a dilution of 1:5000 in PBS with 5% skim milk, pH 7.4. Membranes were washed with PBS, pH 7.4 before the addition of the chemiluminescent substrate (Super Signal West Pico) according to the manufacturer's instructions and exposed to Hyperfilm ECL (Amersham).
3. Results and Discussion
We developed a sandwich ELISA to detect and quantify LukS-PV in culture supernatants of S. aureus strains. We produced rabbit and mouse antisera using rLukS-PV as the immunogen. Preliminary experiments indicated that both antisera detected purified native and rLukS-PV by ELISA when LukS-PV was directly coated on microtiter plates (data not shown). To detect LukS-PV in S. aureus culture supernatants, rabbit antiserum bound to a microtiter plate was used to capture LukS-PV from CA-MRSA bacterial cultures and mouse antiserum was used to detect the LukS-PV captured by the rabbit antiserum. Surprisingly, positive signals were detected with supernatants from both pvl+ CA-MRSA isolate LAC (Figure 1; Filled squares) and pvl- CA-MRSA isolate SA-109 (Figure 1; Filled circles).
Figure 1. Sandwich ELISA to detect LukS-PV in bacterial cultures.
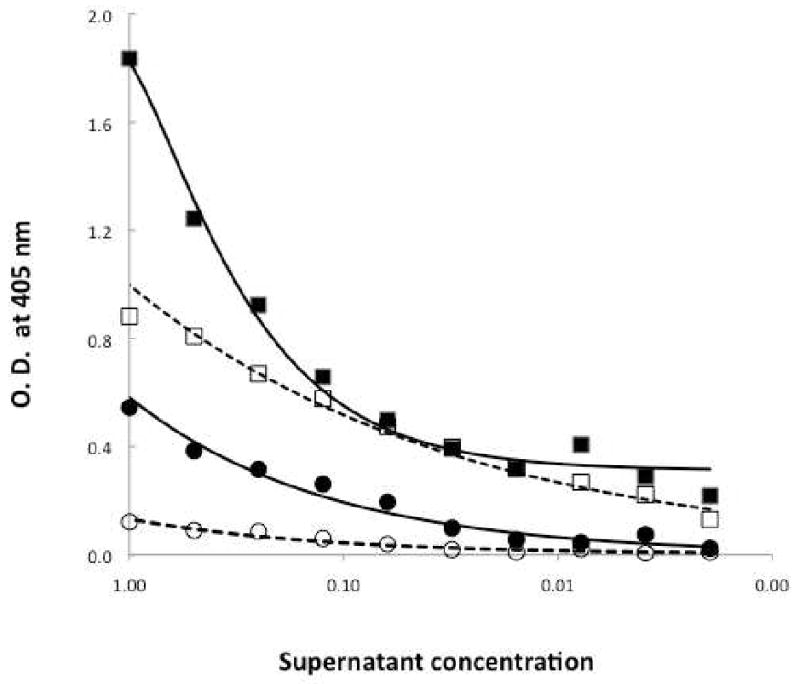
Microtiter plates coated with rabbit anti-LukS-PV were incubated overnight with diluted culture supernatants from SA-109 (circles) and LAC (squares) CA-MRSA bacterial strains (closed symbols, solid lines), or microtiter plates coated with rabbit anti-LukS-PV were incubated for 20 min with 5 mM DEPC and incubated overnight with diluted culture supernatants (open symbols, dashed lines). The plates were incubated with mouse anti-LukS-PV followed by goat anti-mouse IgG conjugated to alkaline phosphatase and developed by adding disodium p-nitrophenyl phosphate. The optical density (O.D.) was determined at 405 nm.
Spa secreted by S. aureus strains is known to cause interference in immunological assays (Guidry et al., 1991). To determine if Spa was responsible for the positive signal with SA-109, ELISA was repeated using chicken anti-protein A conjugated to HRP as the detecting antibody. Spa was detected in both LAC and SA-109 strains (Figure 2A; Without DEPC). These results suggest that Spa in the bacterial cultures binds to the rabbit antibodies immobilized on the ELISA plates. It is reasonable to assume that Spa in SA-109 binds to the coating rabbit antibody, and the detecting murine antibodies bind to the captured Spa yielding a positive signal.
Figure 2. Sandwich ELISA to detect LukS-PV and Spa in presence of DEPC.
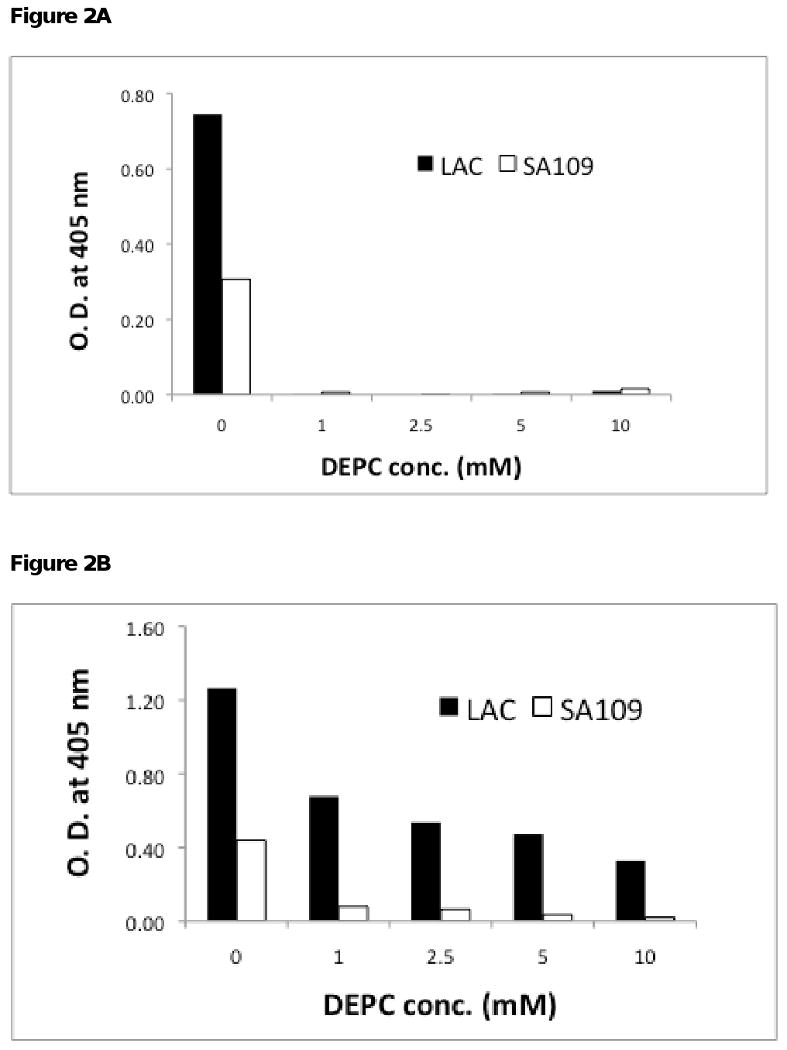
A, Microtiter plates coated with rabbit anti-LukS-PV were incubated overnight with diluted culture supernatants. Spa bound to the capturing rabbit antibody was detected by chicken anti-protein A conjugated to HRP. B, LukS-PV was detected by carrying out the ELISA as described in Figure 1.
To confirm the presence of Spa, culture supernatants from pvl+ MRSA strains SA-123, SA-112 and LAC and pvl- MRSA strain CA-109 were analyzed by SDS-PAGE and Western blotting. Rabbit anti-LukS-PV detected a band of MW 32 kD corresponding to LukS-PV. In addition, an intense band of MW 42 kD, consisting of a doublet, was also detected in culture supernatants of SA-123, SA-109 and LAC (Figure 3A). The 42 kD band was absent in SA-112, which is known to produce very little if any Spa. When the proteins were probed with chicken anti-protein A, the 42 kD band was detected in LAC, SA-109, SA-123, but not in SA-112 (Figure 3B) indicating that protein A secreted by the CA-MRSA strains is bound by rabbit anti-LukS-PV.
Figure 3. Western blot analysis of culture supernatants from four clinical CA-MRSA isolates.
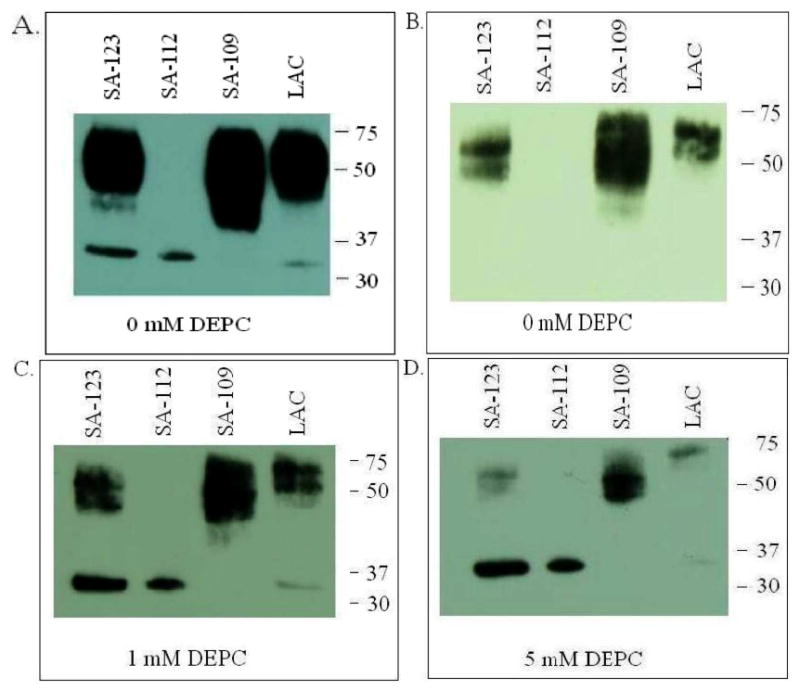
The isolates vary in the presence of genes for PVL as well as the production of protein A: pvl+ SA-123, pvl+ LAC producing low levels of pvl, pvl- SA-109 and pvl+ SA-112 producing very low levels of Spa. Proteins were separated by SDS/PAGE in 4-20% Tris-glycine gels and transferred to nitrocellulose. Nonspecific sites were blocked by incubating the blots in PBS containing 5% skim milk (PBS-milk). Blots were first incubated with (A) rabbit anti-LukS-PV in PBS-milk, (C) rabbit anti-LukS-PV mixed with 1 mM DEPC, or (D) rabbit anti-LukS-PV mixed with 5 mM DEPC, followed by goat anti-rabbit antibodies conjugated to HRP. (B) protein A was detected using chicken anti-protein A conjugated to HRP. Blots were developed by incubation with Super Signal West Pico Chemiluminescent Substrate and exposure on CL-XPOsure Film.
Haake and coworkers have shown that DEPC can inhibit Spa binding to IgG (Haake et al., 1982). When microtiter plates coated with rabbit anti-LukS-PV were incubated with 5 mM DEPC, no signal was observed with spa+/pvl- CA-MRSA strain SA-109, whereas attenuated positive signal was observed with spa+/pvl+ LAC culture supernatants (Figure 1; dashed lines). Pre-treatment of rabbit antiserum with 5 mM DEPC for 10 min was sufficient to inhibit binding of Spa to rabbit IgG. The interference due to Spa was inhibited with increasing concentrations of DEPC with 1, 5 and 10 mM DEPC inhibiting 75%, 90% and 95%, respectively (Figure 2B).
To determine if DEPC is effective in Western blotting under denaturing conditions, culture supernatants from spa+/pvl- and spa+/pvl+ CA-MRSA isolate culture supernatants were analyzed by SDS-PAGE and Western blotting using rabbit anti-LukS-PV as the detecting antibody in the presence of DEPC followed by goat anti-rabbit conjugated to HRP (Figure 3). The binding of anti-LukS-PV to Spa was inhibited with increasing concentrations of DEPC with 5 mM DEPC inhibiting 95% of binding (Figure 3C & 3D). The inhibition was dependent on the amount of Spa produced by the bacterial strain.
Spa binding is mediated through an exposed histidine residue at position 435 in human IgG (Deisenhofer, 1981). It has been proposed that DEPC interferes with the binding of Spa to histidine-435 in human IgG (Schroder et al., 1987). Our results indicate that DEPC prevents the direct binding of Spa to anti-LukS-PV. In addition to histidine, lysine, tyrosine, serine and threonine residues can be modified by DEPC (Hnizda et al., 2008; Mendoza and Vachet, 2008). Because these residues may be found in the antigen-binding site of antibodies, we determined if the presence of DEPC interferes with the binding of LukS-PV to anti-LukS-PV in the sandwich ELISA. To directly compare the binding characteristics, the bacterial culture supernatants and YCP media were dialyzed against PBS. The binding of anti-LukS-PV pre-incubated with 5 mM DEPC to rLukS-PV added to SA-109 (pvl-) culture supernatant (Figure 4; dashed line and open diamonds) was similar to the binding of anti-LukS-PV to LukS-PV in culture media YCP (Figure 4; solid squares). However, when the binding of anti-LukS-PV pre-incubated with 5 mM DEPC to rLukS-PV added to SA-109 (pvl-) culture supernatant (Figure 4; dashed line and open diamonds) was compared to the binding of anti-LukS-PV to rLukS-PV added to SA-109 (pvl-) culture supernatant (Figure 4; solid line and open triangles), DEPC appeared to have a small impact on the binding of anti-LukS-PV to LukS-PV. Furthermore, when the culture supernatants were analyzed by Western blot, the band corresponding to LukS-PV in supernatant from LAC strain appeared less intense in the presence of DEPC, confirming that pretreatment of DEPC has a small impact on LukS-PV binding to its cognate antibody. It is interesting to note that the difference in binding of anti-LukS-PV to LukS-PV becomes significant only at a lower dilution of the supernatants indicating that there may be a subset of the polyclonal antibody pool affected by DEPC treatment. In this case, a higher concentration of polyclonal antiserum or a carefully chosen monoclonal antibody might be better reagents for the sandwich ELISA.
Figure 4. Binding characteristics of anti-LukS-PV pre-incubated with DEPC to rLukS-PV in YCP media and bacterial culture supernatants.
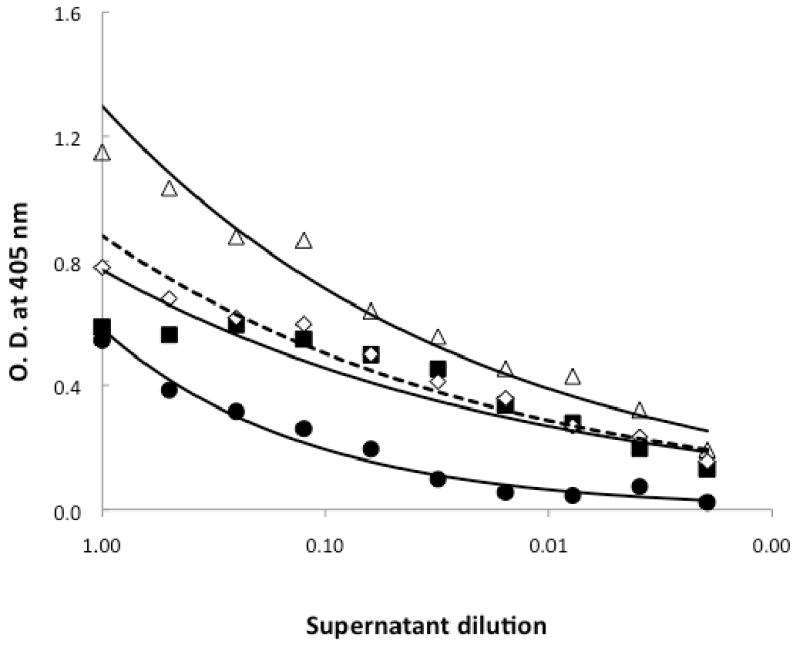
ELISA was carried out as described in Figure 1. Shown is the binding of 2 μg/ml rLukS-PV in dialyzed YCP media to anti-LukS-PV (closed squares, solid lines) and the binding of 2 μg/ml rLukS-PV in dialyzed SA109 supernatant to DEPC-treated anti-LukS-PV (dashed line, open diamonds). Controls shown are dialyzed SA-109 culture supernatants binding to anti-LukS-PV (solid circles) and dialyzed SA-109 culture supernatants containing 2 μg/ml rLukS-PV binding to anti-LukS-PV (open triangles).
In summary, we have developed a sandwich ELISA to detect and quantify LukS-PV in culture supernatants of S. aureus strains. We show that DEPC can be used to inhibit binding of rabbit IgG to protein A, known to cause interference in immunological techniques. We have used this sandwich ELISA to quantify LukS-PV secreted by S. aureus strains in presence of different antibiotics and confirmed the quantification results by determining the relative amounts of LukS-PV secreted using Western blots (manuscript in preparation). Using DEPC should prove useful in using antibody detection methodologies, such as ELISA and Western blotting, when protein A may cause interference.
Acknowledgments
This work was supported by National Institute of Health grant AI071025 to DOB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berdal BP, Olsvik O, Omland T. A sandwich ELISA method for detection of Staphylococcus aureus enterotoxins. Acta Pathol Microbiol Scand B. 1981;89:411–415. [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20:2361–70. [PubMed] [Google Scholar]
- Fey H, Burkhard G. Measurement of staphylococcal protein A and detection of protein A-carrying staphylococcus strains by a competitive ELISA method. Journal of Immnological Methods. 1981;47:99–107. doi: 10.1016/0022-1759(81)90260-x. [DOI] [PubMed] [Google Scholar]
- Freed RC, Evenson ML, Reiser RF, Bergdoll MS. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol. 1982;44:1349–55. doi: 10.1128/aem.44.6.1349-1355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding JW. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–53. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Guidry AJ, Squiggins KE, Vann WF, Westhoff DC. Prevention of nonspecific binding of immunoglobulin to Staphylococcus aureus protein A in ELISA assays. J Immunol Methods. 1991;143:159–65. doi: 10.1016/0022-1759(91)90041-d. [DOI] [PubMed] [Google Scholar]
- Haake DA, Franklin EC, Frangione B. The modification of human immunoglobulin binding to staphylococcal protein A using diethylpyrocarbonate. J Immunol. 1982;129:190–2. [PubMed] [Google Scholar]
- Hahn IF, Pickenhahn P, Lenz W, Brandis H. An avidin-biotin ELISA for the detection of staphylococcal enterotoxins A and B. J Immunol Methods. 1986;92:25–9. doi: 10.1016/0022-1759(86)90499-0. [DOI] [PubMed] [Google Scholar]
- Hnizda A, Santrucek J, Sanda M, Strohalm M, Kodicek M. Reactivity of histidine and lysine side-chains with diethylpyrocarbonate -- a method to identify surface exposed residues in proteins. Journal of Biochemical and Biophysical Methods. 2008;70:1091–1097. doi: 10.1016/j.jbbm.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Hoffman WL, Ruggles AO, Tabarya D. Chicken anti-protein A prevents Staphylococcus aureus protein A from binding to human and rabbit IgG in immunoassays and eliminates most false positive results. J Immunol Methods. 1996;198:67–77. doi: 10.1016/0022-1759(96)00152-4. [DOI] [PubMed] [Google Scholar]
- Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- Ladhani S, Robbie S, Garratt RC, Chapple DS, Joannou CL, E RW. Development and Evaluation of Detection Systems for Staphylococcal Exfoliative Toxin A Responsible for Scalded-Skin Syndrome. Journal of Clinical Microbiology. 2001;39:2050–2054. doi: 10.1128/JCM.39.6.2050-2054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza VL, Vachet RW. Protein surface mapping using diethylpyrocarbonate with mass spectrometric detection. Analytical chemistry. 2008;80:2895–2904. doi: 10.1021/ac701999b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost G, Cribier B, Couppie P, Petiau P, Supersac G, Finck-Barbancon V, Monteil H, Piemont Y. Panton-Valentine leucocidin and gamma-hemolysin from Staphylococcus aureus ATCC 49775 are encoded by distinct genetic loci and have different biological activities. Infect Immun. 1995;63:4121–9. doi: 10.1128/iai.63.10.4121-4129.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogemond V, Da Costa Castro JM, Delaunay T, Cazaux C, Guinet RM. Protein A insensitive ELISA detection of staphylococcal enterotoxin B. Lett Appl Microbiol. 1991;13:175–8. doi: 10.1111/j.1472-765x.1991.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Sasso EH, Silverman GJ, Mannik M. Human IgA and IgG F(ab′)2 that bind to staphylococcal protein A belong to the VHIII subgroup. Journal of Immunology. 1991;147:1877–1883. [PubMed] [Google Scholar]
- Schroder AK, Nardella FA, Mannik M, Johansson PJ, Christensen P. Identification of the site on IgG Fc for interaction with streptococci of groups A, C and G. Immunology. 1987;62:523–527. [PMC free article] [PubMed] [Google Scholar]


