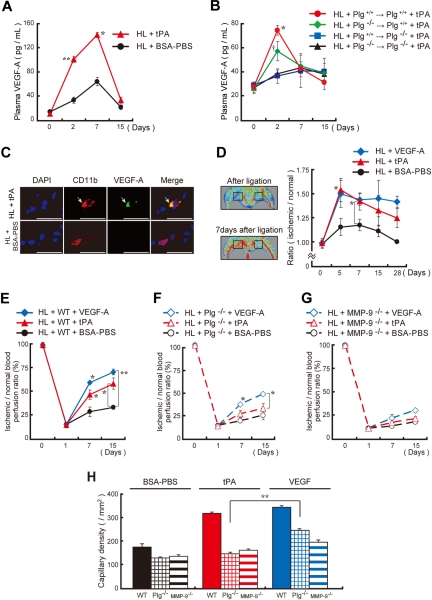
Figure 4.
Administration of recombinant VEGF-A partially rescues the angiogenic defect observed in Plg- and MMP-9–deficient mice. (A-B) Plasma VEGF-A levels were analyzed in tPA-treated and untreated HL-induced C57BL/6 (A) and chimeric (B) mice (n = 3 per group). Chimerism was achieved as follows: Lethally irradiated Plg+/+ or Plg−/− mice received a transplant with Plg+/+ or Plg−/− donor BM cells. Six weeks later, HL ischemia–induced chimeric mice were treated intraperitoneally with/without tPA. Asterisks and single dagger indicate a significant difference between tPA-treated and BSA-PBS–treated groups. (C) Immunohistochemical staining for CD11b and VEGF-A of ischemic muscle tissue derived from tPA-treated and vehicle-treated HL ischemic mice. Arrows indicate CD11b+ VEGF-A+ cells (bar = 25 μm). Images were acquired with 40×/0.75 NA and a Carl Zeiss KS400 camera, and analyzed using Axio Vision (Carl Zeiss). (D-H) HL-induced wild-type control (D-E,H), Plg−/− (F,H), and MMP-9−/− (G,H) mice were injected intraperitoneally daily with tPA, mVEGF-A, or BSA-PBS from day 0 until day 2. (D) Functional perfusion measurements of the collateral region with the use of a laser scan at the indicated time points in wild-type animals. Representative laser Doppler perfusion images are shown of the regions analyzed. Blood perfusion (red to yellow) was augmented in tPA-treated mice compared with vehicle-injected controls (green to blue = reduced perfusion signal). (E-G) Blood flow recovery was determined by laser Doppler perfusion imaging in the ischemic limbs (n = 3; *P < .05, **P < .001). (H) Capillary density was evaluated on CD31-stained sections 15 days after ligation (n = 3; **P < .001). Asterisks indicate a significant difference between the indicated groups. Error bars represent SEM.