Figure 4. Functional and physical interaction of NFATc and EGR-1.
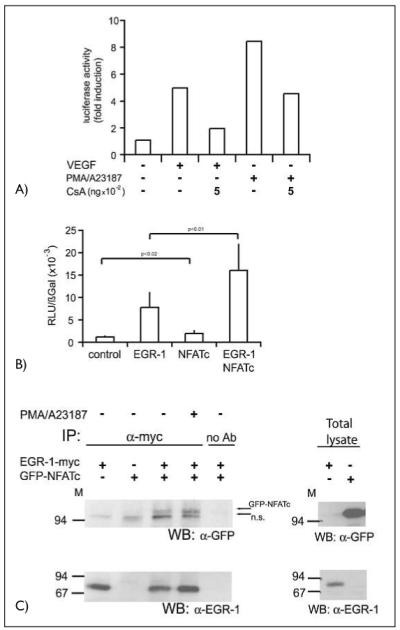
A) A reporter gene construct containing a 330-bpTF promoter element was transfected into HUVEC. Sixteen h post transfection the cells were starved in M199 medium with 2% serum for at least 8 h. The cells, in part pretreated with CsA as indicated, were then stimulated with VEGF (100 ng/ml) or a combination of PMA (10 nM) and A23187 (2.5 μM) for further 6 h. Then the cells were lysed and luciferase activity was measured using a dual-luciferase reporter assay system. A representative experiment of three performed in duplicates is shown. B) Endothelial cells were transfected with a tissue factor reporter gene construct containing the wild-type –330 bp promoter sequence. Cotransfection was performed with either an EGR-1– or an NFATc overexpression construct or a combination of both. Twenty-four h post transfection the cells were harvested, lysed and luciferase activity determined. Luciferase values were normalized to β-Gal expression from a cotransfected CMV-βGal construct. Mean values ± SD obtained from three independent experiments performed in duplicates are displayed. C) Two hundred ninety-three HEK cells were cotransfected with overexpression plasmids for myc-tagged EGR-1 and GFP-NFATc fusion protein. Cells were harvested without or after treatment with a combination of PMA (10 nM) and A23187 (2.5 μM) for 60 min. Cells and immunoprecipitations were performed with rabbit polyclonal anti-myc antibodies and protein A sepharose beads overnight. Equal aliquots of the immunoprecipitates or aliquots of the total cellular lysates were then loaded on 7.5% SDS-PAGE and probed with α-GFP or α-EGR-l antibodies. The positions of the GFP-NFATc and of a non-specific band (n.s.) are indicated by arrows.
