Figure 1. Metabolic alterations promoting tumor cell aerobic glycolysis and associated therapeutic targets.
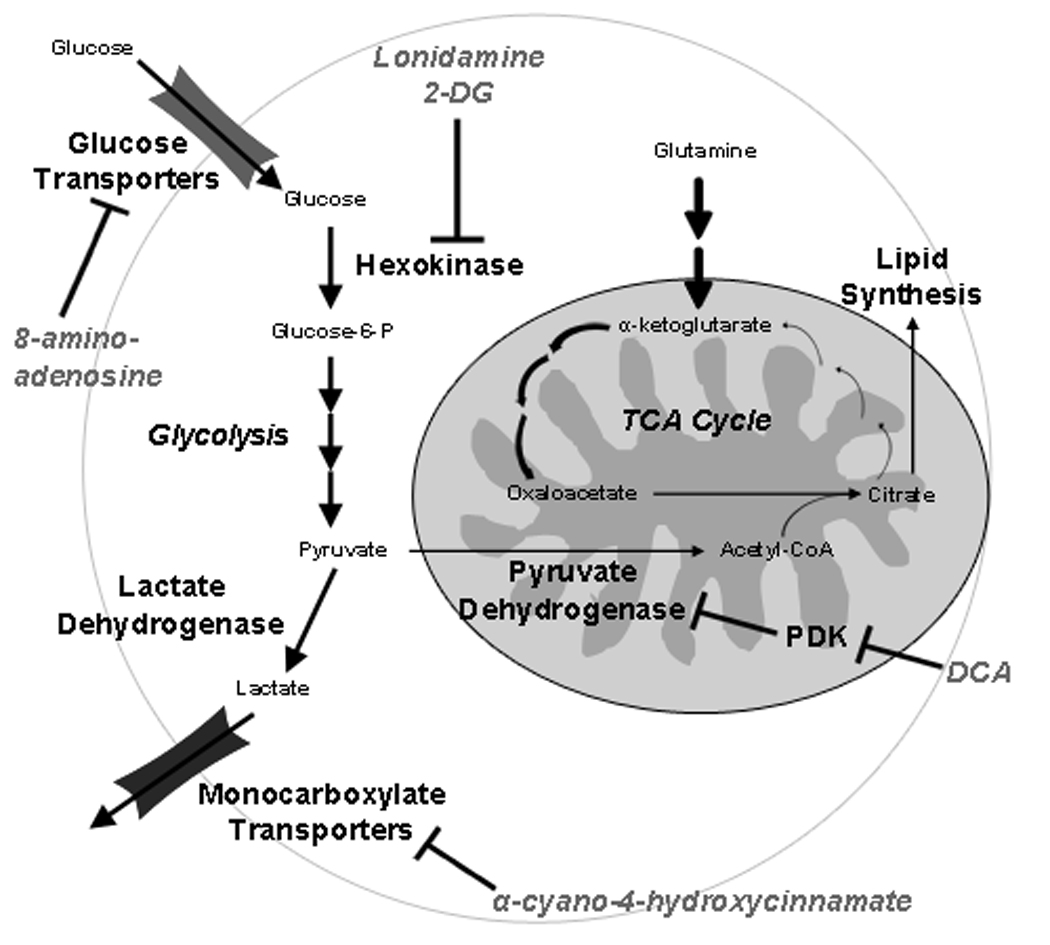
Tumor cells increase glucose consumption through enhanced expression and activity of glucose transporters and hexokinase. Hexokinase carries out the initial phosphorylation of glucose, which is required to retain glucose molecules within the cell. In normal cells, glucose is converted to pyruvate in a series of reactions in the cytosol. Pyruvate, the end product of these reactions, enters the mitochondria and is converted to acetyl-CoA, which is used in the tricarboxylic acid cycle (TCA) cycle to drive ATP synthesis via oxidative phosphorylation. In tumor cells, glucose-derived pyruvate is preferentially converted to lactate in the cytosol and subsequently extruded from the cell through monocarboxylate transporters. Pyruvate molecules which do enter the mitochondria in this context are used largely to fuel a truncated TCA cycle wherein citrate is siphoned out of the mitochondria to stimulate fatty acid biosynthesis. Glutamine can then be used to replenish subsequent metabolites in the TCA cycle through conversion to α-ketoglutarate. Specific enzyme targets for lonidamine, 2-deoxyglucose (2-DG), 8-amino-adenosine, dichloroacetate (DCA), and α-cyano-4-hydroxycinnamate are indicated. Bold arrows indicate enhanced activity in tumor cells.
