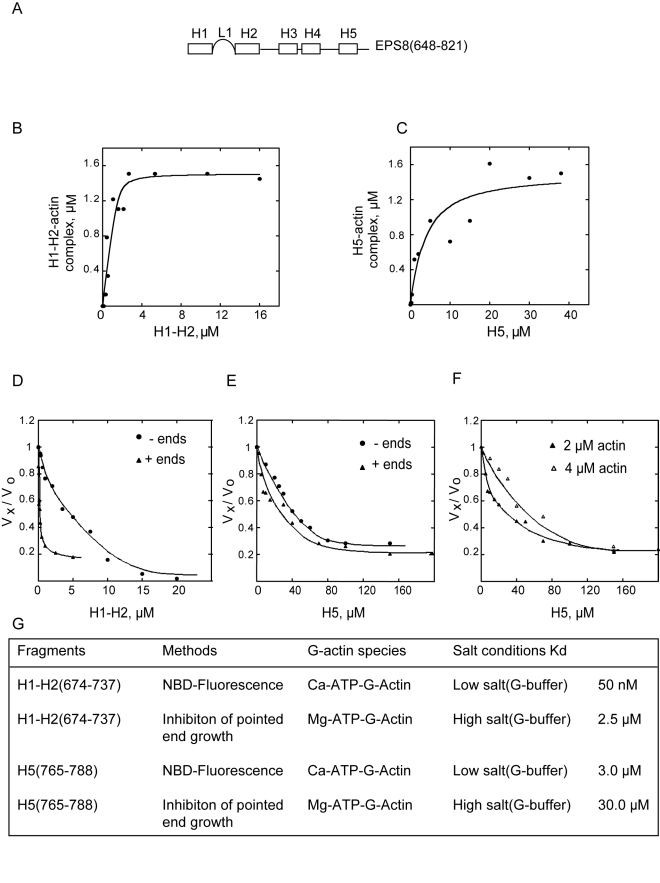
Figure 2. Eps8(648–821) possess two monomeric actin binding surfaces: H1–H2 and H5.
(A) A schematic representation of the secondary structure organization of Eps8 actin binding domain. H1, H2, H3, H4, and H5 indicate stretches of amino acids that adopt an helical conformation; L1 is the 20 amino acid-long linker connecting H1 to H2 (see for additional details and amino acids sequence Figure S1B and S1C). (B, C) H1–H2 and H5 bind G-actin in a concentration-dependent manner. The change in fluorescence of 1.5 µM NBD-labeled-actin was measured at different concentrations of either H1–H2 (B) or H5 (C), in low salt (G buffer). Symbols indicate data; solid lines indicate fitted binding curves for a complex with 1∶1 stoichiometry. (D, E) H1–H2 and H5 inhibit barbed and pointed end elongation rates with different thermodynamic constants. The rate of elongation was measured from pointed ends (circles), using gelsolin-actin seeds (5 nM) and 2 µM of G-actin (10% pyrenyl-labeled), or from barbed ends (triangles), using spectrin-actin seeds (2 nM), in the presence of increasing concentrations of H1–H2 (D) or H5 (E), as indicated. Rates are normalized taking as 100% the rate of elongation measured in the absence of H1–H2 or H5. Kds are reported in the text. (F) H5 sequesters G-actin but does not cap filaments ends. The rate of elongation from barbed ends was measured using actin seeds and 2 µM (closed triangles) or 4 µM (opened triangles) of G-actin (10% pyrenyl-labeled) in the presence of increasing concentrations of H5. A shift toward the left of the rate of elongation by H5 as the concentration of G-actin used increased indicates that stoichiometric concentrations of H5 with respect to actin are required for the inhibition. (G) Summary of the equilibrium parameters for binding of H1–H2 and H5 to monomeric actin. The values were obtained as described in the methods from the experimental curves show in (A–F). Please note that the Kd obtained from the inhibition of pointed end growth experiments reflects the binding affinity of the tested helices for monomeric actin leading to its sequestration and were performed at physiological salt condition.