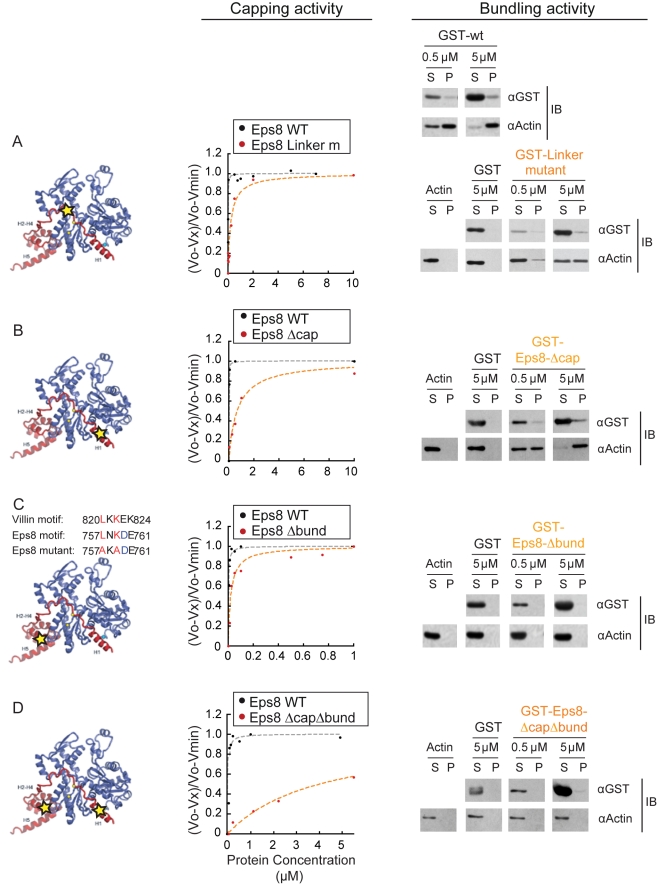
Figure 6. Eps8 capping and bundling activities can be dissected.
(A) The barbed end capping and bundling activity of Eps8 requires an intact H1-to-H2 Linker (Linker mutant) region. Left: the position of critical, mutated residues of the H1-to-H2 Linker in the modeled complex Eps8(648–821):G-actin are indicated by a star. Middle: the rates of elongation from barbed ends using spectrin-actin seeds and 2 µM of G-actin (10% pyrenyl-labeled) in the presence of increasing concentrations Eps8(648–821)-R706A-F708A-(Eps8-Linker mutant) (orange line) with respect to Eps8(648–821)(Eps8-WT) (gray lines) are shown. Rates are normalized taking as 100% the rate of elongation from barbed ends measured in the absence of Eps8-WT. Symbols indicate data; line indicates fitted binding curves for a complex with 1∶1 stoichiometry. The curve is calculated using Equation 1. The kinetic constants (Kcap) of barbed end inhibition for Eps8-Linker mutant = 180 nM; Eps8-WT = 9 nM. Right: the F-actin bundling ability GST-fused Eps8-WT or Eps8-Linker mutant was determined by co-sedimentation assays. F-actin (1 µM) was incubated either alone or in the presence of the indicated concentrations of GST, as control, or GST-fused WT or mutated Eps8(648–821). The mix was subjected to centrifugation at 10,000 g for 30 min. Aliquots of the pellet (P) and supernatants (S) were analyzed by immunoblotting with the antibodies indicated on the right. (B) The barbed end capping but not the bundling activity of Eps8 requires an intact H1 amphipathic helix. Left: the position of critical, mutated residues of the H1 in the modeled complex Eps8(648–821):G-actin are indicated by a star. Middle: the rates of elongation from barbed ends using spectrin-actin seeds in the presence of increasing concentrations of Eps8-WT (gray lines) or Eps8(648–821)-V689D -L693D (Eps8-Δcap) (orange line) are shown. The Kcap of the Eps8-Δcap = 700 nM. Right: the F-actin bundling ability of the indicated Eps8-Δcap was determined as described in (A). (C) The bundling, but not the capping, activity of Eps8(648–821) is mediated by a Villin-like motif in the globular, helical domain. Left: the critical, mutated residues in the Villin-like motif of Eps8(648–821) are indicated in red, and their position in the modeled complex Eps8(648–821):G-actin is indicated by a star. Middle: the rates of elongation from barbed ends in the presence of increasing concentrations of Eps8(648–821)-L757A-K759A (Eps8-Δbund) (orange line) with respect to Eps8-WT (gray line), obtained as described in 6A, are shown (Kcap of Eps8-Δbund = 20 nM). Right: the F-actin bundling ability of GST-Eps8-Δbund was determined as in 6A. (D) Actin capping (middle) and bundling (right) of Eps8(648–821) are impaired by a mutation in the helix H1 and in the Villin-like motif (Eps8-ΔcapΔbund), whose position within the complex Eps8(648–821):G-actin is shown on the left.