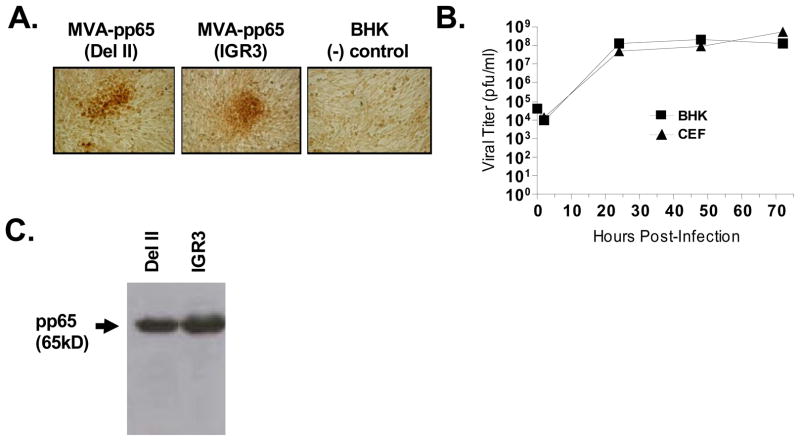
Figure 2. Viability and expression levels of rMVA-pp65-IGR3.
(A) Expression of transgene product (CMV-pp65) during screening process of rMVA-pp65-IGR3. Following 5 rounds of screening, immunohistochemical staining specific for CMV-pp65 was done on BHK-21 cells infected with recombinant MVA containing the CMV-pp65 expression cassette inserted into either the del II site or the IGR3 site. Uninfected BHK-21 cells were used as a (−) staining control. (B) Growth kinetics of rMVA-pp65-IGR3 in BHK-21 and CEF cells. BHK-21 or CEF cells (4×106/100mm dish) were infected with 4×104 pfu (MOI=0.01) of rMVA-pp65-IGR3. Cell lysates were generated 2, 24, 48, and 72 hours after infection and used for viral titer by immunohistochemical staining. (C) Western blot analysis of transgene expression from a rMVA-pp65-IGR3 isolate after purification. Total cell lysates were generated from BHK-21 cells infected with either purified rMVA-pp65-del II or rMVA-pp65-IGR3 and 30 μg of total lysate were loaded for western blot analysis. Details of the approaches can be found in Materials and Methods.