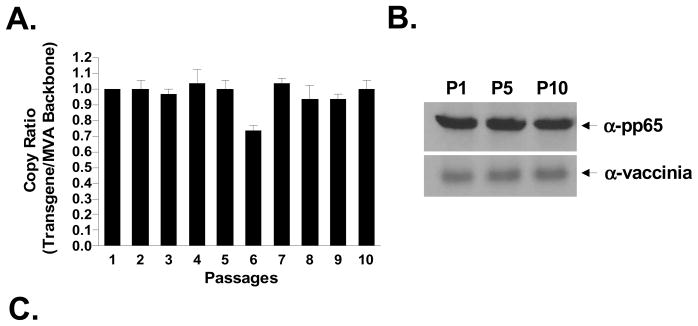
Figure 3. Genetic stability during passage of recombinant MVA-pp65-IGR3.
(A) Following purification of rMVA-pp65-IGR3, transgene stability was determined by quantitative PCR (ABI 7300 Real-Time PCR System) during 10 passages on BHK-21 cells. Copy ratios are expressed as transgene copies (CMV-pp65) to MVA backbone copies. The analysis was repeated 3 times and shown is the mean of all 3 runs. Error bars represent standard error of the mean (SEM). Details of the approach are described in Materials and Methods. (B) Western blot analysis of CMV-pp65 at passages 1, 5, and 10. Equal amounts of total protein lysate (30 ug, Bradford assay) were loaded for each sample. Additional western blot against vaccinia protein using an anti-BR8 vaccinia mAb was performed as a loading control. (C) Using primers specific for the IGR3 flanking region (Table 1), PCR analysis was performed using a Perkin Elmer 9600 Gene Amp PCR system to detect for the presence or absence of wildtype (wt) IGR3 sequence (~300 bp) and the CMV-pp65 expression cassette (~2.7 kb) in viral genomic DNA isolated from rMVA-pp65-IGR3 at passages 1, 5, and 10.