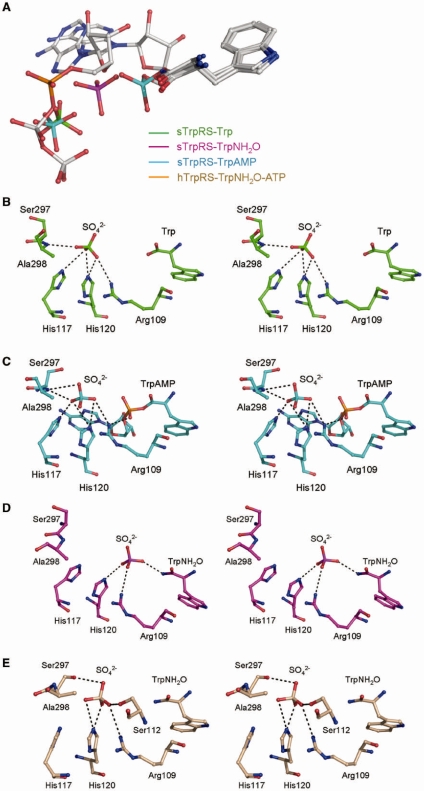
Figure 2.
Sulfate binding in the sTrpRS complexes. (A) Structural comparison of the bound ligands among the sTrpRS complexes and the hTrpRS–TrpNH2O–ATP complex. There is a sulfate ion bound at each active site of the three sTrpRS complexes. Analyses of the sTrpRS complexes and the hTrpRS–TrpNH2O–ATP complex show that during the amino activation reaction the Trp moieties remain at similar positions, while the α-phosphate appears to approach Trp from a distant position observed in the hTrpRS–TrpNH2O–ATP complex (orange) to an intermediate position similar to that of the sulfate ion in monomer A of the sTrpRS–TrpNH2O complex (magenta), and to a final position in the sTrpRS–TrpAMP complex (cyan) when the reaction is completed. The sulfate ions in the sTrpRS–Trp (green) and sTrpRS–TrpAMP (cyan) complexes occupy a position equivalent to that of the β-phosphate of ATP in the hTrpRS–TrpNH2O–ATP complex. For clarity, all the ligands are colored in gray except the α-phosphate of ATP and the sulfate ions. (B–E) Stereoviews showing the interactions of the sulfate ions with the surrounding residues at the active site in (B) the sTrpRS-Trp complex, (C) the sTrpRS–TrpAMP complex, and (D) monomer A and (E) monomer B of the sTrpRS–TrpNH2O complex, respectively.