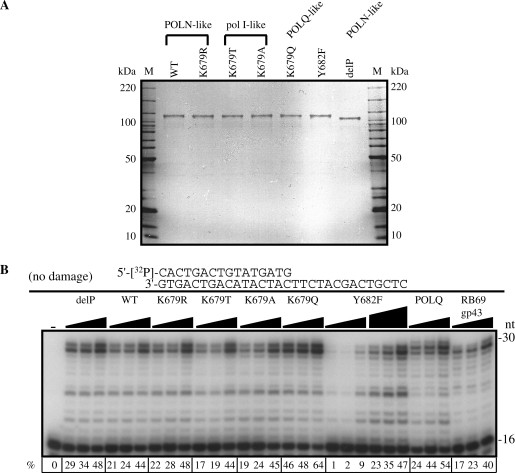
Figure 2.
Activity of wild-type (WT) and mutant POLN on a nondamaged DNA template. (A) Purified POLN derivatives (300 ng) and molecular weight markers were separated by electrophoresis in a 4–15% SDS–polyacrylamide gradient gel and stained with colloidal Coomassie Brilliant Blue G-250. Substituted residues in POLN derivatives are shown in Figure 1. delP is POLN with a short truncation of the C-terminal proline-rich tail. (B) DNA polymerase activities of POLN derivatives. Increasing amounts of delP, WT, K679R, K679T, K679A and K679Q (6, 12 and 23 nM), Y682F (6, 12, 23, 29, 58 and 115 nM), POLQ (3, 6 and 12 nM), RB69 gp43 (2.5, 5 and 10 pM) were incubated with the 5′-32P-labeled primer templates indicated beside the panel in the presence of all 4 nt at 37°C for 10 min. The first lane contained no enzyme. The percentage (%) of the product extension from the primer is shown below each lane. The specific activity of POLN is 127 U/mg, delP is 125 U/mg, K679R is 116 U/mg, K679T is 112 U/mg, K679A is 124 U/mg, K679Q is 153 U/mg and Y682F is 32 U/mg. One unit is defined as 10 nmol of dTTP incorporated into poly(dA)/oligo(dT)10:1 template at 37°C for 30 min.