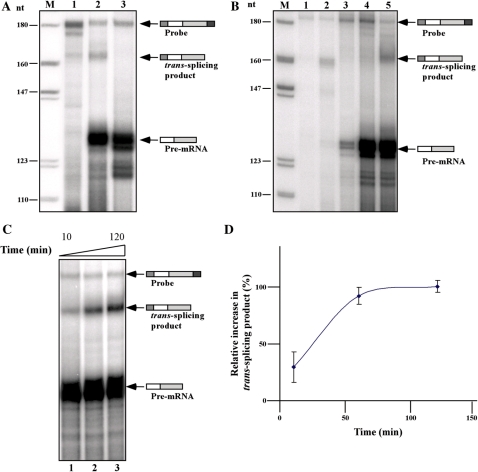
Figure 3.
The trans-splicing reaction is heat-inactivated, requires ATP and is time-dependent. (A) Trans-splicing in heat-inactivated extracts. For heat inactivation, the trans-splicing extract was incubated for 30 min at 55°C. The trans-splicing product was compared to that produced in untreated extract. Total RNA was prepared from the reaction and subjected to RNAse protection as described in ‘Materials and Methods’ section. Lane contents are as follows: 1, RNase protection assay with RNA (30 µg) from pNS21-TIR transgenic cells; 2, In vitro trans-splicing reaction using untreated extract (control); 3, In vitro trans-splicing reaction using heated-inactivated extract. (B) Co-transcriptional trans-splicing and ATP requirement of the trans-splicing reaction. In vitro trans-splicing was performed in the presence of 0.8 µg pNS-21-TIR plasmid DNA as described in the ‘Results’ section. For ATP depletion, the ATP regenerating system was omitted from the reaction. After incubation at 28°C for 1 h, total RNA was prepared and subjected to RNase protection as described in ‘Materials and Methods’ section. Lane contents are as follows: 1, Antisense probe (500 c.p.m.); 2, total RNA (30 µg) from the transgenic pNS21-TIR cells; 3, RNA from extracts containing plasmid pNS21-TIR; 4, RNA from trans-splicing reaction lacking the ATP regenerating system; 5, RNA from trans-splicing performed under optimal conditions (control). M- DNA marker, labeled pBR322 DNA MspI digest. (C) Kinetics of the in vitro trans-splicing reaction. In vitro trans-splicing was conducted under standard conditions but for different durations (10′, 60′ and 120′). RNA was subjected to RNase protection as described in ‘Materials and Methods’ section. (D) Quantitation of the time-dependent splicing from three independent experiments.