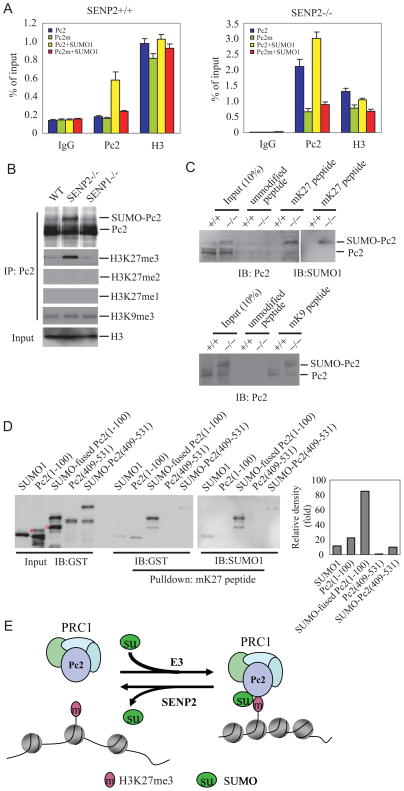
Figure 6. SUMOylation increases binding affinity of Pc2 to H3K27me3.
(A) Mutation of SUMOylation site decreases the ability of Pc2 to occupy the promoter of Gata4. Two antibodies (anti-Flag and -H3) were used in the qChIP assays using SENP2+/+ or −/− MEF cells transfected with indicated plasmids. Data are shown in means±s.d. of three independent transfection experiments.
(B) The immunoprecipitates with Pc2 (IP) from chromatin fraction of SENP2+/+ (WT), SENP2−/−, or SENP1−/− MEF cells were detected by immunoblotting with anti-Pc2 and anti-H3K27me3, H3K27me2, H3K27me1, and H3K9me3 (IB). Chromatin fractions were immunoblotted (IB) with anti-H3 as input.
(C) SUMOylated Pc2 binds to tri-methylated H3K27, but not H3K9, with higher affinity than un-conjugated Pc2. Biotinylated histone H3 peptides that were either unmodified or tri-methylated on K27 (upper panel) or on K9 (bottom panel), were incubated with the chromatin fractions from SENP2+/+ (WT) or SENP2−/− (Mut) MEFs in the presence of streptavidin-conjugated sepharose beads. The precipitates were detected by immunoblotting (IB) with anti-Pc2 or anti-SUMO1.
(D) SUMOylation facilitates binding of Pc2 chromodomain to tri-methylated H3K27. Biotinylated histone H3 peptides that were tri-methylated on K27 (mK27), were incubated with GST-SUMO1, GST-Pc2(1-100), GST-SUMO1-fused Pc2(1-100), GST-Pc2(409-531), and SUMOylated GST-Pc2(409-531) recombinant proteins in the presence of streptavidin-conjugated sepharose beads. Inputs and precipitates by mK27 peptides as detected with anti-GST were shown in the left panel. The precipitates by mK27peptidea as detected with anti-SUMO1 were shown in the middle panel. The relative binding affinity was shown as relative fold change of signal density of precipitates detected by anti-GST and standardized with input (Right panel). “*” indicates a non-specific band. “ ” indicates a degraded band.
” indicates a degraded band.
(E) A model depicting the role of SENP2 in the regulation of PRC1 recruitment to H3K27me3 through controlling the SUMOylation status of Pc2. E3 ligase for Pc2 may be Pc2 itself or an undefined E3 ligase.