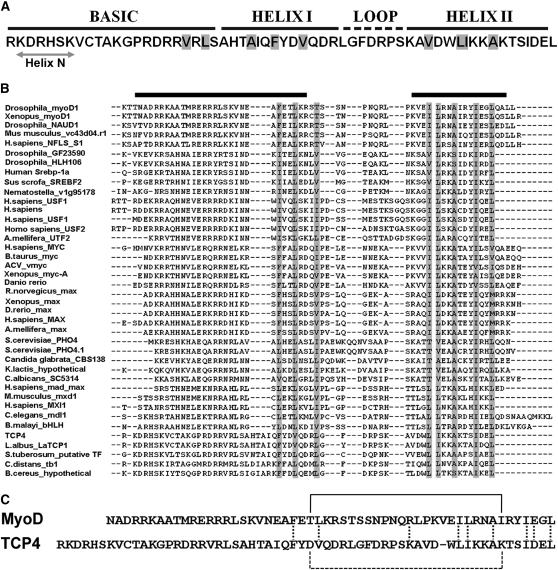
Figure 3.
Sequence Comparison between TCP and bHLH Domains.
(A) Sequence of the TCP4 domain and the predicted bHLH secondary structure are shown. A predicted short helix at the N terminus (helix N) is indicated. The residues predicted to be buried are shaded in gray.
(B) TCP4 sequence profile (see Results) was aligned with the profiles generated for the bHLH proteins of known three-dimensional structure. The residues that are fully conserved or substituted for similar amino acids are highlighted in gray. The helical regions for the MyoD structure are shown by black bars on the top of the alignment.
(C) Sequence alignment of MyoD and TCP4 DNA binding domains, highlighting the similarities in the C-terminal HLH region. The residue conservation in the HLH domains is shown by vertical dotted lines. van der Waals contact between Thr-33 and Ile-56 of MyoD (solid line) and a probable salt bridge interaction between corresponding residues Asp-75 and Lys-97 in TCP4 (broken line) are shown. No significant sequence similarity is observed in the N-terminal basic region.