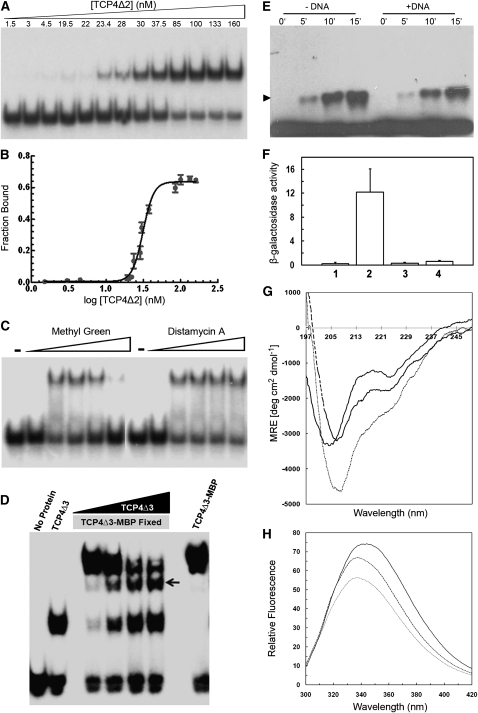
Figure 5.
Biochemical Characterization of the TCP Domain.
(A) EMSA gel showing that a fixed amount of radioactively labeled target oligo incubated with increasing amount of purified TCPΔ2 retards progressively increasing amount of oligos. Numbers above the gel indicate concentration of dimer TCPΔ2.
(B) Fraction of bound oligo was quantified from (A) and plotted as a function of dimer protein concentration. Analysis of this data by Hill equation yielded an equilibrium dissociation constant (Kd) of 31.3 ± 2.2 nM.
(C) EMSA gel performed with purified TCP4Δ1 and target DNA probe in the presence of increasing amount of methyl green or distamycin A. Free oligo in the absence or the presence of 500 μM dye are shown as (−) and (+), respectively.
(D) Detection of homodimerization of the TCP4 DNA binding domain by EMSA. A band of intermediate mobility (indicated by an arrow) appeared when purified TCP4Δ3 (7 kD) and TCP4Δ3-MBP (50.2 kD) were incubated together with the 32P-labeled consensus oligos. The intermediate band increased in intensity when increasing amounts of TCP4Δ3 were incubated with a fixed amount of TCP4Δ3-MBP.
(E) Immunoblot of purified hexa-His-tagged TCP4Δ1 incubated with 0.01% of glutaraldehyde for increasing time points and probed with anti-(His)6 antibody. The monomeric TCP4 protein is seen here as a continuous band at the base of the denaturing polyacrylamide gel and the dimer band (arrowhead) of increasing intensity formed with increasing time of incubation. Addition of target oligo to the reaction mixture (+DNA) slightly inhibited dimer formation.
(F) The strength of protein–protein interaction, as performed by yeast two-hybrid assay, has been shown as β-galactosidase activity. Each experiment was repeated thrice, and the standard errors are shown as error bars along the y axis. The bait in all the assays is TCP4Δ1. Proteins used as prey are no protein (1), TCP4 (2), I93N (3), and I100T (4) mutants.
(G) CD spectra of 5 μM purified TCP4Δ2 with 0 μM (solid line), 1 μM (dashed line) and 5 μM (dotted line) target DNA. The spectra are corrected for the contribution of buffer (for solid line) or buffer plus oligo (for dashed and broken lines).
(H) Relative fluorescent intensity spectra of 5 μM purified, truncated TCP4 in the absence (solid line) or presence of 1 μM (dashed line) and 5 μM (dotted line) target oligo.