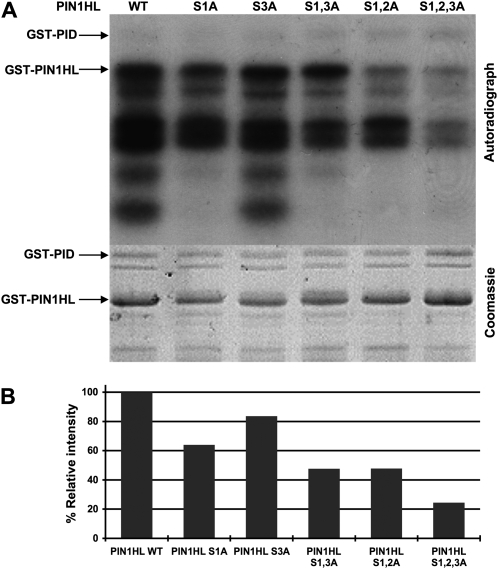
Figure 2.
Ser Residues in Three Conserved Motifs within the PIN1HL Are Phosphorylated by PID in Vitro.
(A) In vitro assay of phosphorylation by GST-PID kinase using wild-type GST-PIN1HL and mutant protein substrates in which the indicated Ser residues (S1, S2, or S3) were replaced with Ala residues (A). The positions of GST-PID and the full-length GST-PIN1HL are indicated in the autoradiograph (top panel) and the Coomassie-stained gel (bottom panel). Autophosphorylation of GST-PID can be observed in the top panel. Under our experimental conditions, Escherichia coli–purified GST-PIN1HL was not stable, resulting in a reproducible pattern of degradation bands. The Coomassie blue–stained gel was used as a control for protein loading.
(B) Quantitative assessment of the in vitro phosphorylation assay in (A). The phosphorylation intensity is expressed as the percentage of phosphorylation relative to the wild-type GST-PIN1HL protein. Numbers were corrected for protein loading based on analysis of the Coomassie blue–stained blot.