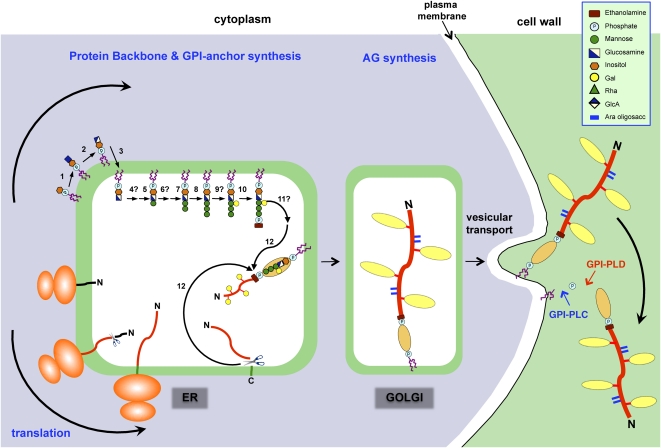
Figure 3.
Proposed mechanism for the synthesis and addition of a GPI anchor to an AGP. The addition of a GPI anchor to an AGP likely occurs in several phases, beginning with the synthesis of the GPI moiety on the cytoplasmic surface of the ER. The protein backbone is inserted into the ER cotranslationally, and eventually the two processes converge. The GPI anchor synthesis pathway shown is a composite of that established for mammals and yeast (Orlean and Menon, 2007; Kinoshita et al., 2008) based on the structure of the pear GPI anchor (Fig. 1A). A similar pathway likely exists in plants, since orthologs of the mammalian and yeast genes have been found in plants (Table I). Proteins involved in the biosynthesis pathway (numbered) are summarized in Table I. In both mammals and yeast, any branching glycosylation of the trimannosyl core occurs between steps 8 and 10, and although galactosylation is plant specific, it is presumed to occur at the same stage (step 9). Since substitution of inositol (step 6) and the trimannosyl core (step 11) with acyl and ethanolamine phosphate residues, respectively, has not been demonstrated in plant GPIs, these steps are not shown here, although, interestingly, BLAST searches have identified putative acyl transferases (Table I). Following concomitant removal of the C-terminal GPI signal sequence and addition of the GPI anchor (step 12), numerous Pro residues present within the AGP backbone are hydroxylated to Hyp. Substitution of these Hyp residues with AG chains probably begins within the ER and is completed within the Golgi network. AGPs are transported to the plasma membrane via vesicular transport, where they are either temporarily anchored to the plasma membrane before being released by phospholipases (GPI-PLC and GPI-PLD) or endocytosed. This model is modified from Schultz et al. (1998).