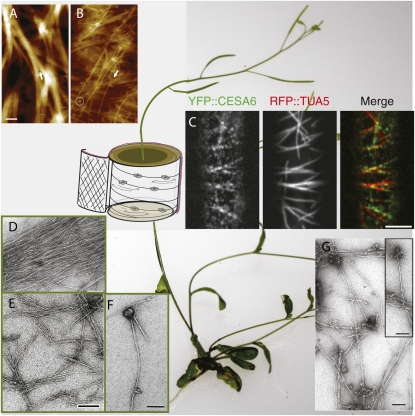
Figure 1.
Emerging tools for cellulose analysis in plant cell walls. The background is set with the model plant Arabidopsis; a simple schematic is overlaid on the stem to indicate various facets of cell wall cellulose synthesis. A, AFM height image of fibril structures in the elongating primary cell wall of maize contained primarily macrofibrils that split at the end (indicated by the arrow). B, The mature primary cell wall contained both small macrofibrils in the upper-most layer that can also split at the end (indicated by the arrow) and parallel-arranged single elementary fibrils. Particle-like features were clearly seen and were typically associated with the macrofibrils (indicated by the circle). Bar in A = 100 nm. C, Confocal image of upper hypocotyl cells of transgenic plants expressing YFP::CESA6, red fluorescent protein (RFP)::TUA5 (tubulin), and the overlay of the two compatible fluorophors in a merged imaged reveals the coincidence of CESA with microtubules. Bar = 5 μm. D to F, Electron micrographs of cellulose microfibrils extracted from primary walls of blackberry cells (D and E) and of cellulose synthesized in vitro (F) using detergent extracts as a source of enzyme. The cellulose from cell walls was observed before (D) and after (E) treatment with the Updegraff reagent (Lai Kee Him et al., 2002). A cellulose microfibril synthesized in vitro associated to a globular particle, which most likely corresponds to the enzyme complex (not treated with the Updegraff reagent; F). G, Electron micrograph of cellulose microfibrils synthesized in vitro by DRMs isolated from hybrid aspen cells grown as suspension cultures (negative staining with 2% uranyl acetate). The inset shows the magnification of putative DRM structures that carry the active cellulose synthase machinery and from which cellulose microfibrils are being formed. Bars in E to G = 0.1 μm.