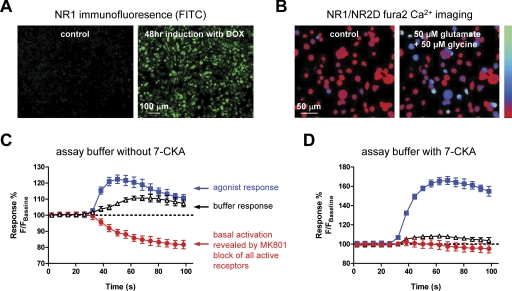
Fig. 1.
A, induction of NR1 visualized by immunofluorescence using anti-NR1 antibody after 48 h of doxycycline (DOX) treatment. Secondary antibody is fluorescein isothiocyanate-conjugated. Control refers to cells that were not treated with doxycycline and therefore do not express NR1. B, Fura-2 imaging of a BHK-21 cell line expressing NR1/NR2D during challenge with glutamate and glycine (50 μM each). Ratiometric images at 340/380-nm excitation and 510-nm emission for Fura-2 before (control) and after challenge with glutamate and glycine are shown. The bar (left) shows Ca2+ signal intensity ranging from 0 to 4 mM Ca2+ (bottom is 0 mM Ca2+). C and D, time course of fluorescence responses in the NR1/NR2D cell line in the absence and presence of 30 μM of the competitive glycine antagonist 7-CKA in the assay buffer. Agonist (blue squares), buffer (black triangles), or (+)-MK801 (red circles) were added to the well at time 20 s. The data points are displayed as percentage of baseline fluorescence 100% × F/Fbaseline, where F is the fluorescence measured after addition to the well, and Fbaseline is the average fluorescence measured before addition to the well. Data points are mean ± S.D. of four to five wells. Final agonist concentrations were 100 μM glutamate and 1 mM glycine. The uncompetitive open channel blocker (+)-MK801 (10 μM) was used to inhibit any receptors that were active before addition of the agonist and thereby reveal the basal activity of the receptors. The basal activity was virtually absent in the assay when 30 μM 7-CKA was added to the assay buffer.