Figure 1.
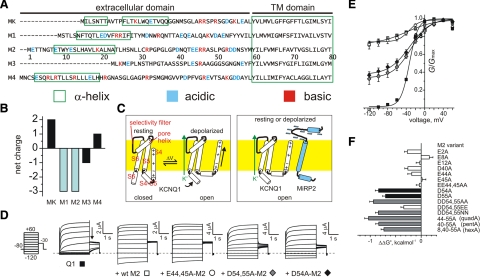
MiRP2 D54 and D55 stabilize the open state of KCNQ1. A) Sequences of the predicted extracellular and transmembrane domains of the human KCNE family, aligned gap-free by their transmembrane domains. MK, MinK; M1–M4, MiRPs 1–4. Numbering is for human M2. Charged residues are highlighted. Green boxes, positioning of α-helices according to NMR structure determination for MK (28) or prediction using the PHD program for M1-M4 (29). Dashes, before start of methionine. B) Net extracellular domain charge for MinK (MK) and MiRPs 1–4 (M1–M4). C) Cartoons of voltage-dependent activation of homomeric KCNQ1 (left) vs. constitutive activation of MiRP2-KCNQ1 (right), indicating a hypothetical model for stabilization of KCNQ1 S4 in the activated state by some or all of the 8 MiRP2 extracellular domain acidic residues (blue minus symbols). Yellow, plasma membrane. For clarity, S4–S6 from only one KCNQ1 α subunit is shown. D) Exemplar current traces recorded in oocytes expressing KCNQ1 alone (Q1) or with wild-type (wt), EE44,45AA-MiRP2, DD54,55AA-MiRP2, or D54A-MiRP2 (M2) as indicated. Inset: currents were recorded by TEVC, using the standard voltage family protocol. Dashed line indicates 0 current level. E) Mean normalized conductance (G/Gmax, measured at arrow in D); channels and symbols as in D. n = 36 (Q1); 44 (+wt M2); 15 (E44,45A); 10 (D54,55A); 13 (D54A). Error bars = se. F) Mean ΔΔG° for the closed to open transition, calculated from macroscopic conductance plots as in E and for other single and multiple MiRP2 (M2) mutants with KCNQ1; n = 8–44. Error bars = se.