Abstract
Males and females of almost all organisms exhibit sexual differences in body size, a phenomenon called sexual size dimorphism (SSD). How the sexes evolve to be different sizes, despite sharing the same genes that control growth and development, and hence a common genetic architecture, has remained elusive. Here, we show that the genetic architecture (heritabilities and genetic correlations) of the physiological mechanism that regulates size during the last stage of larval development of a moth, differs between the sexes, and thus probably facilitates, rather than hinders, the evolution of SSD. We further show that the endocrine system plays a critical role in generating SSD. Our results demonstrate that knowledge of the genetic architecture underlying the physiological process during development that ultimately produces SSD in adults can elucidate how males and females of organisms evolve to be of different sizes.
Keywords: sexual dimorphism, physiology, quantitative genetics, body size
1. Introduction
Sexual size dimorphism (SSD), a difference in body size between males and females, is common in animals (Fairbairn 1997, 2007). The direction and magnitude of SSD varies considerably across taxa (Andersson 1994; Stillwell et al. 2010). For example, males are often larger than females in mammals, whereas females are often larger than males in insects (Lindenfors et al. 2007; Stillwell et al. 2010). Most of this variation in SSD is owing to three major sources of selection: (i) fecundity selection favouring large females, (ii) sexual selection favouring large males, and (iii) selection for a shorter duration of growth and/or a faster growth rate and hence small size in both sexes (Stillwell et al. 2010). However, despite extensive research on evolutionary explanations for how selection can generate SSD, a major paradox remains unresolved: how do males and females evolve to be different in body size despite sharing the same genes that regulate growth and development (Badyaev 2002; Fairbairn & Roff 2006; Stillwell et al. 2010)? The variation in SSD we observe among organisms shows that this constraint can be overcome (Stillwell et al. 2010), but a sufficient explanation is still lacking.
Evolutionary quantitative genetic studies often measure the extent to which a shared genome between sexes can hinder the evolution of SSD. These empirical studies generally demonstrate that the genetic correlation (which measures a shared genome) for body size between the sexes is near 1.0 and that the heritabilities (which measure genetic variation and the ability to respond to selection) are similar between sexes (Lande 1980; Reeve & Fairbairn 1996; Roff 1997; Kruuk et al. 2008; Poissant et al. 2010). This indicates that selection on body size in one sex will produce an equal response to selection in the opposite sex. Therefore, we should expect little to no SSD in organisms, even with substantial selection differences between sexes. Selection experiments support this prediction: attempts to change the magnitude of SSD via artificial selection on body size of males and females generally results in little to no change in SSD (Reeve & Fairbairn 1996; Delph et al. 2004). How then do we reconcile the theoretical prediction that the evolution of SSD will be constrained by a shared genome with the empirical evidence of the remarkable variety of dimorphisms we see among organisms? One caveat of nearly all prior studies is that they focused almost exclusively on the genetic architecture of adult body size in predicting the evolution of SSD. However, understanding the evolution of SSD requires knowledge of how SSD forms during growth and development, because even if the ultimate target of sex-specific selection is adult body size, the proximate target is the sex-specific developmental process that produces adult SSD (Reeve & Fairbairn 1996; Badyaev 2002; Poissant & Coltman 2009; Poissant et al. 2010; Stillwell et al. 2010). Consequently, the inconsistencies between patterns of SSD found in nature and predictions from quantitative genetic theory may be resolved by understanding the genetic architecture of the growth process that ultimately produces SSD.
There are only three ways males and females can become different in body size during development: the sexes must differ in their size at hatching, growth rate and/or duration of growth (Stillwell et al. 2010). In insects, sex differences in size at hatching do not appear to produce the female-biased SSD (i.e. larger females) observed in most insect species (Esperk et al. 2007; Stillwell et al. 2010). This female-biased SSD is partly explained by females growing faster than males in some species of insects (Blanckenhorn et al. 2007; Stillwell et al. 2010). However, in many insects females prolong their growth period and thus increase their size (Esperk et al. 2007). The duration of the growth period is determined by when growth ceases. How body size is regulated by the cessation of growth has only recently been identified (Davidowitz & Nijhout 2004; Nijhout et al. 2006).
The developmental process that determines the final size of an adult insect is best understood in the hawkmoth Manduca sexta. Davidowitz & Nijhout (2004) describe the physiological mechanism that terminates growth in the last stage of development (where 90% of mass is accumulated and thus where SSD probably develops) and hence regulates body size: over 95 per cent of the variation in body size is explained by this mechanism (D'Amico et al. 2001; Davidowitz & Nijhout 2004; Nijhout & Davidowitz 2009). In brief, three factors regulate body size in M. sexta: the growth rate, the critical weight (which measures the cessation of juvenile hormone (JH) secretion from the corpora allata) and the interval to the cessation of growth (ICG; which measures the time interval between the critical weight and the secretion of the ecdysteroids that regulate pupation and metamorphosis). The duration of the growth period is determined by the critical weight and the ICG. The nonlinear interaction between these three factors determines the final size of the larva and hence the adult moth (Nijhout et al. 2006, 2010).
Like most insects, M. sexta exhibits SSD with females larger than males (Davidowitz et al. 2004). Here, we use the detailed knowledge of the developmental process that determines body size in this system to investigate how the genetic architecture underlying this physiological mechanism enables the evolution (Davidowitz et al. 2005) of dimorphism in adults. Specifically, we test the hypothesis that females become larger than males because they have a faster growth rate, a larger critical weight and a longer ICG than males. In addition, we test the hypotheses that the narrow-sense heritabilities for these three traits will be higher in females than in males and that the genetic correlation between sexes for these traits is less than 1.0, allowing females to evolve independently during development to produce the evolution of female-biased SSD in adults.
2. Material and methods
(a). Study population
The M. sexta population used in this study was outcrossed from laboratory colonies from Duke University, the University of Arizona and the University of Washington. To minimize maternal effects, the data were collected eight generations after the colonies were outcrossed.
(b). Experimental design
To initiate the experiment, single male and female pupae that were ready to eclose were chosen at random from the outcrossed colony and placed together in a no. 420 brown paper bag (11.2 l) to create full-sibling families. Upon eclosion, moths were given ad libitum 25 per cent (v/v) sucrose solution as a nectar source in a white cone drinking cup to simulate a natural hawkmoth pollinated flower (Raguso & Willis 2002), a Genpak waterpik (approx. 133 ml) for humidity and a Styrofoam platform (approx. 50 cm2) with an ethanol leaf extract (1∶5 v/v) to mimic the leaves of the native host plant (Datura wrightii: Solanaceae) to stimulate female oviposition. The nectar, waterpik and oviposition platform were replenished daily within each bag. Eggs were collected daily from all bags. On all parents and offspring we measured the pupal mass, larval peak mass and the development time of 5th instar larvae. Peak larval mass and pupal mass largely determine adult size (Davidowitz et al. 2004). The growth rate, the critical weight and the ICG were calculated as below.
All individuals (parents and offspring) were reared on a 16∶8 (L∶D) photoperiod at 25°C on the standard rearing diet ad libitum (100% diet in Davidowitz et al. 2003). Larvae in instars 1–4 were reared individually in clear plastic cups (29.6 ml) with a perforated lid for gas exchange. Last (5th) instar larvae were transferred into 266 ml cups with straw slits for gas exchange. Upon the initiation of pupation (Davidowitz et al. 2004), wandering larvae were placed in 266 ml cups with potting soil.
(c). Physiological calculations
All calculations presented here were carried out on 1222 offspring from 170 families, with 6–10 (mode = 8) offspring per family.
Larval peak mass (g) was measured 3 h before the onset of the scotophase prior to wandering at which time body mass reaches its maximum (G. Davidowitz 2003, unpublished data). Pupal mass (g) was measured on pupae seven days following the onset of wandering, which is the first day that pupae can be handled without damage. Growth rate (g d−1) was measured for the 24 h period between the third- and second-to-last days of larval growth. This ensured all individuals were measured during their linear phase of growth (Nijhout et al. 2006). A regression of the last three days of growth showed that the mean coefficient of determination (r2) was 0.99, indicating that the growth rate during this part of larval growth was linear and constant. Development time (day) of 5th (last) instar larvae was measured as the number of days between the molt to the 5th instar and wandering. During this instar 90 per cent of larval growth occurs (Davidowitz et al. 2004).
It is not possible to directly measure the critical weight and the ICG on a single individual as the critical weight and the ICG are population level traits (D'Amico et al. 2001; Davidowitz et al. 2003, 2004). In this colony (population), the critical weight is 7.0 g and the population ICG is 1.92 days. It is possible, however, to estimate these traits on individuals indirectly as cwi = pmi − (icgp * gri), where cwi is the individual critical weight, pmi is the individual peak mass, icgp is the population level estimate of ICG (1.92 days) and gri is the individual growth rate. The mean individual critical weight (cwi) of all offspring calculated with the above equation was not significantly different from the population critical weight (cwp = 7.0 g); mean offspring individual critical weight = 7.0 g, t1221 = −0.10, p = 0.92.
Similarly, the individual ICG was calculated as icgi = (pmi − cwp)/gri, where icgi is the individual ICG, pmi is the individual peak mass as above, cwp is the population critical weight (7.0 g) and gri is the individual growth rate as above. The mean offspring ICG (icgi) was 1.94 days which was not significantly different from the population mean ICG of 1.92 days (t1221 = 1.77, p = 0.08).
(d). Genetic estimates and analyses
Narrow-sense heritabilities (h2) and between-sex genetic correlations (rA) for development time, peak larval mass, pupal mass, growth rate, critical weight and the ICG were estimated using parent–offspring regression with the variance component procedure in SAS (PROC VARCOMP, REML estimation; Fry 1992; Astles et al. 2006). h2's for each sex were estimated as twice the covariance among sons and sires and twice the covariance among daughters and dams. rA's were calculated as σfemales–males2/σfemales σmales, where σfemales–males2 is the family main effect for the complete mixed model (separate covariances were obtained for sons and dams/daughters and sires, with the average of the two used as the numerator for the calculation of rA; Roff 1997) and σfemales and σmales are square roots of the family main effect for the two reduced models, one for each sex (the covariance among sons and sires and the covariance among daughters and dams). Standard errors of genetic parameters were obtained by jackknifing families (Roff & Preziosi 1994; Windig 1997). Because jackknifing produces a normal distribution of these genetic parameters, we used t-tests to compare means between the sexes. Two-sample t-tests were used to test whether h2 differed between sexes. For the critical weight and the ICG, we used a one-sample t-test to test whether female h2 differed from 0 (male h2 were 0 for these traits with a s.e. = 0). A one-sample t-test was used to test whether rA's were significantly different from 1.0.
3. Results
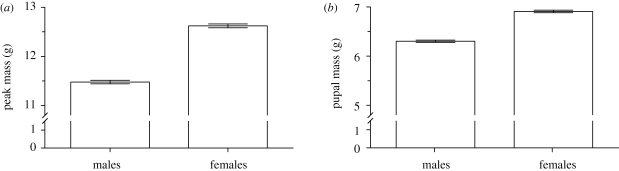
Females had approximately 10 per cent larger larval peak mass than males (sex effect in ANOVA: F1,1220 = 387, p < 0.0001; figure 1a), which resulted in female pupae that were approximately 10 per cent larger than male pupae (F1,1220 = 321, p < 0.0001; figure 1b). Larval peak mass and pupal mass determine adult size (Davidowitz et al. 2004). Although we did not measure adult size directly in this study, in another study, under identical rearing conditions, SSD in larval and pupal mass resulted in SSD in adult body mass (females were 19% larger than adult males, F1,183 = 30.4, p < 0.0001; mean ± s.e.m.; females: 2.91 g ± 0.06; males: 2.45 g ± 0.06).
Figure 1.
(a) Peak larval mass and (b) pupal mass of males and females of the hawkmoth Manduca sexta. Error bars show standard errors.
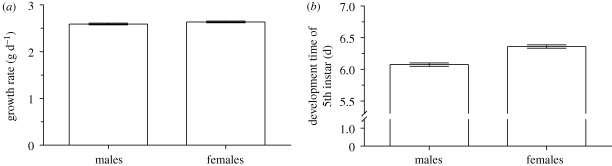
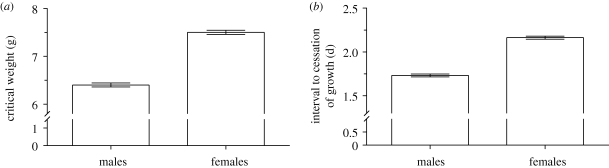
The sexes had similar growth rates (less than 2% difference between sexes; F1,1220 = 2.90, p = 0.09; figure 2a), indicating that the SSD in M. sexta cannot be attributed to a sex difference in the growth rate. Rather, the SSD is owing to a difference between males and females in the duration of growth; the total development time of 5th (last) instar females (including time to reach the critical weight and the ICG) was 5 per cent longer than for males (F1,1220 = 60.5, p < 0.0001; figure 2b). This sex difference in the duration of growth is owing to a sex difference in the critical weight and the ICG. Females had approximately 17 per cent larger critical weight than males (F1,1220 = 346, p < 0.0001; figure 3a), indicating that females initiate the process of metamorphosis later than males. Females also had approximately 25 per cent longer ICG than males (F1,1220 = 317, p < 0.0001; figure 3b), suggesting that females take longer to clear JH from their haemolymph than do males.
Figure 2.
(a) Growth rate and (b) the development time of 5th instar males and females of the hawkmoth Manduca sexta. Error bars show standard errors.
Figure 3.
(a) Critical weight and the (b) interval to cessation of growth (ICG) of males and females of the hawkmoth Manduca sexta. Error bars show standard errors.
Interestingly, females had considerably higher heritabilities (h2) than males for larval peak mass (t169 = 2.17, p = 0.03), critical weight (t169 = 3.41, p = 0.0008) and the ICG (t169 = 3.28, p = 0.0013; table 1). However, the between-sex genetic correlations (rA) were not significantly different from 1.0 for peak mass, pupal mass, growth rate and 5th instar development time, while the rA's were undefined for the critical weight and the ICG (table 1).
Table 1.
Narrow-sense heritabilities (h2) of males and females and between-sex genetic correlations (rA) for larval peak mass, pupal mass, growth rate, critical weight, the interval to cessation of growth (ICG) and 5th instar development time of the hawkmoth Manduca sexta. (Standard errors were obtained by a jackknifing routine created by the authors in SAS. Two-sample t-tests were used to compare means except where noted. See text for explanation on the calculation of genetic parameters. Note: rA's were undefined for the critical weight and the ICG owing to zero covariances for males.)
| males |
females |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h2 | s.e. | h2 | s.e. | t | p | rA | s.e. | ta | p | |
| peak mass | 0.48 | 0.07 | 0.73 | 0.09 | 2.17 | 0.03 | 0.72 | 0.17 | 1.62 | 0.11 |
| pupal mass | 0.28 | 0.08 | 0.49 | 0.09 | 1.74 | 0.08 | 0.86 | 0.23 | 0.61 | 0.54 |
| growth rate | 0.30 | 0.08 | 0.24 | 0.08 | 0.46 | 0.64 | 0.53 | 0.37 | 1.25 | 0.21 |
| 5th instar dev. time | 0.33 | 0.07 | 0.15 | 0.07 | 1.76 | 0.08 | 1.14 | 0.48 | 0.29 | 0.77 |
| critical weight | 0 | 0 | 0.29 | 0.08 | 3.41b | 0.0008 | — | — | — | — |
| ICG | 0 | 0 | 0.27 | 0.08 | 3.28b | 0.0013 | — | — | — | — |
aA one-sample t-test was used to test the means against the hypothesis that the mean = 1.
bA one-sample t-test was used to test the means against the hypothesis that the mean = 0.
4. Discussion
Our results demonstrate that the physiological mechanism that regulates body size probably facilitates, rather than hinders, the evolution of SSD in M. sexta. The rA's between sexes for all the traits we examined were 1.0 or undefined (table 1), suggesting that the evolution of SSD should be constrained. However, despite having an rA = 1.0, the sexes can evolve independently, even if selection acts equally on both sexes; if the sexes have unequal h2 (Cheverud et al. 1985; Lynch & Walsh 1998), the response to selection (assuming all else being equal) will be greater in one sex versus the other owing to higher genetic variation in one sex. Here we found that females had a considerably higher h2 for larval peak mass, critical weight and the ICG compared with males. Although the rA's for the critical weight and the ICG were undefined, the response to selection will be greater in females than in males because of the unequal h2, even if we assume that rA = 1.0 for these traits. This indicates that females can evolve to be larger in this species through a sex difference in the genetic architecture of larval size and in the physiological mechanism that controls larval size. Furthermore, our study indicates that understanding the evolution of SSD by focusing exclusively on the genetic architecture of adult SSD may be misleading because the proximate target of selection is the developmental process in the larvae that ultimately produces SSD in the adults.
Our results also show that the female-biased SSD of M. sexta is proximately generated by females extending their duration of growth via a larger critical weight and longer ICG during ontogeny than males. Although previous studies have shown that the sexes can achieve differences in size through differences in development time in insects, our study is the first, to our knowledge, to reveal the underlying physiological mechanisms involved (i.e. the timing of hormonal events that determine when growth ends). Other studies involving the endocrine system support these conclusions, but did not address dimorphism in size directly (Baker et al. 1987; Bhaskaran et al. 1988). Our results on M. sexta strongly suggest that the endocrine system plays a critical role in development of SSD in insects.
Although our findings offer a promising glimpse of how genetic and physiological mechanisms may regulate SSD in insects, the underlying details about how the sexes actually differ in their genetic architecture (e.g. sex-biased gene expression, sex linkage, etc.) and how this is coupled to the endocrine system are not well understood (Stillwell et al. 2010). However, some other studies on M. sexta offer some clues. For example, the black larval mutant of M. sexta is caused by a recessive gene (bl-) that is sex-linked (Safranek & Riddiford 1975; Franks & Lampert 1993). Animals homozygous for this gene are 37 per cent smaller in size, have a 20 per cent lower growth rate and, apparently, have lower titers of JH than wild-type individuals (Safranek & Riddiford 1975; Kramer & Kalish 1984; Orth & Goodman 1995). It is possible that the black mutants are smaller because they have a smaller critical weight and a shorter ICG than the wild types owing to decreased titers of JH and premature cessation of JH secretion (Stillwell et al. 2010). However, future studies need to test the hypothesis that sex-linkage could be responsible for the sex difference in the genetic architecture of larval size, the critical weight and the ICG.
Our study reveals how a sex difference in genetic architecture of a physiological mechanism can facilitate the evolution of SSD in M. sexta, but is it possible that these results are more generally applicable to other insects? The physiological mechanisms that regulate body size in M. sexta are very similar to the physiological mechanisms that regulate body size in Drosophila melanogaster (Edgar 2006; Mirth & Riddiford 2007; Shingleton et al. 2007, 2008). For example, the critical weight and the terminal growth period (similar to the ICG) are essential components of the mechanism that regulates size in D. melanogaster (Edgar 2006; Shingleton et al. 2007). However, there are some differences in the hormonal control of the physiological mechanisms that regulate size in these two species (see Edgar 2006). In addition, there is no information on sex differences in the genetic architecture of physiological mechanisms that regulate body size in D. melanogaster. It is thus difficult to generalize about the role these physiological mechanisms may play in the evolution of SSD in insects until more studies are conducted.
In summary, our study resolves the paradox of how males and females evolve different sizes by demonstrating that the genetic architecture of larval size and the physiological mechanism that regulates size differs between sexes in the last larval stage of M. sexta, thereby allowing the independent evolution of size between males and females. In addition, the sex difference in this physiological mechanism indicates that the endocrine system plays a critical role in the development of adult SSD. Finally, we suggest that focusing on the evolution of SSD at all stages of development will help us better understand how SSD evolves in organisms (Badyaev 2002).
Acknowledgements
We thank Alex Badyaev, Omar Eldakar, Bryan Helm, Kristen Potter and two anonymous reviewers for comments on a previous version of this manuscript. G.D. thanks Alice Levine and Connie Meyers for their unwavering assistance in managing the experiments, and to Jenny Barker, Kacie Bressmer, Tina Ceccato, Erick Chen, Shuang Chen, Ben Collins, Sarah Diamond, Nicole Ferguson, Jenny Graber, Ali Adib Hashemi, Lindsey Halcrow, Briana Horvath, Tuan Khuu, Brianne Kiley, Henry Krigbaum, Jack Lin, Kelly Mackay, Tony Macko, Wendy Mitchell, Jim Pearson, Virginia Pham, Ben Pri-Tal, Mahesha Rajapakse, Rebecca Ruppel, Elliot Saperstein, Thomas Smith, Suzanne Steinberg, Rachel Stewart, David Sung, Becca Trunzo, Ashley Wiede and Maria Williams for assistance in rearing and data collection. This work was supported by grant IBN-0212621 from the National Science Foundation (US) to G.D. and by a Postdoctoral Excellence in Research and Teaching (PERT) fellowship through NIH Training grant no. 1 K12 GM00708 to R.C.S.
References
- Andersson M.1994Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- Astles P. A., Moore A. J., Preziosi R. F.2006Comparison of methods to estimate cross-environment genetic correlations. J. Evol. Biol. 19, 114–122 (doi:10.1111/j.1420-9101.2005.00997.x) [DOI] [PubMed] [Google Scholar]
- Badyaev A. V.2002Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 17, 369–378 (doi:10.1016/S0169-5347(02)02569-7) [Google Scholar]
- Baker F. C., Tsai L. W., Reuter C. C., Schooley D. A.1987In vivo fluctuation of JH, JH acid, and ecdysteroid titer, and JH esterase activity, during development of 5th stadium, Manduca sexta. Insect Biochem. 17, 989–996 (doi:10.1016/0020-1790(87)90108-9) [Google Scholar]
- Bhaskaran G., Sparagana S. P., Dahm K. H., Barrera P., Peck K.1988Sexual dimorphism in juvenile hormone synthesis by corpora allata and in juvenile hormone acid methyl transferase activity in corpora allata and accessory sex glands of some Lepidoptera. Int. J. Invertebr. Rep. Dev. 13, 87–99 [Google Scholar]
- Blanckenhorn W. U., et al. 2007Proximate causes of Rensch's rule: does sexual size dimorphism in arthropods result from sex differences in development time? Am. Nat. 169, 245–257 (doi:10.1086/510597) [DOI] [PubMed] [Google Scholar]
- Cheverud J. M., Dow M. M., Leutenegger W.1985The quantitative assessment of phylogenetic constraints in comparative analyses: sexual dimorphism in body weight among primates. Evolution 39, 1335–1351 (doi:10.2307/2408790) [DOI] [PubMed] [Google Scholar]
- D'Amico L. J., Davidowitz G., Nijhout H. F.2001The developmental and physiological basis of body size evolution in an insect. Proc. R. Soc. Lond. B 268, 1589–1593 (doi:10.1098/rspb.2001.1698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidowitz G., Nijhout H. F.2004The physiological basis of reaction norms: the interaction among growth rate, the duration of growth and body size. Integr. Comp. Biol. 44, 443–449 (doi:10.1093/icb/44.6.443) [DOI] [PubMed] [Google Scholar]
- Davidowitz G., D'Amico L. J., Nijhout H. F.2003Critical weight in the development of insect body size. Evol. Dev. 5, 188–197 (doi:10.1046/j.1525-142X.2003.03026.x) [DOI] [PubMed] [Google Scholar]
- Davidowitz G., D'Amico L. J., Nijhout H. F.2004The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. Res. 6, 49–62 [Google Scholar]
- Davidowitz G., Roff D. A., Nijhout H. F.2005A physiological perspective on the response of body size and development time to simultaneous directional selection. Integr. Comp. Biol. 45, 525–531 (doi:10.1093/icb/45.3.525) [DOI] [PubMed] [Google Scholar]
- Delph L. F., Gehring J. L., Frey F. M., Arntz A. M., Levri M.2004Genetic constraints on floral evolution in a sexually dimorphic plant revealed by artificial selection. Evolution 58, 1936–1946 [DOI] [PubMed] [Google Scholar]
- Edgar B. A.2006How flies get their size: genetics meets physiology. Nat. Rev. Genet. 7, 907–916 (doi:10.1038/nrg1989) [DOI] [PubMed] [Google Scholar]
- Esperk T., Tammaru T., Nylin S., Teder T.2007Achieving high sexual size dimorphism in insects: females add instars. Ecol. Entomol. 32, 243–256 (doi:10.1111/j.1365-2311.2007.00872.x) [Google Scholar]
- Fairbairn D. J.1997Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Annu. Rev. Ecol. Syst. 28, 659–687 (doi:10.1146/annurev.ecolsys.28.1.659) [Google Scholar]
- Fairbairn D. J.2007Introduction: the enigma of sexual size dimorphism. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn D. J., Blanckenhorn W. U., Székely T.), pp. 1–10 New York, NY: Oxford University Press [Google Scholar]
- Fairbairn D. J., Roff D. A.2006The quantitative genetics of sexual dimorphism: assessing the importance of sex-linkage. Heredity 97, 319–328 (doi:10.1038/sj.hdy.6800895) [DOI] [PubMed] [Google Scholar]
- Franks D. L., Lampert E. P.1993The inheritance of cuticular coloration in the tobacco hornworm (Lepidoptera: Sphingidae). J. Entomol. Sci. 28, 96–101 [Google Scholar]
- Fry J. D.1992The mixed-model analysis of variance applied to quantitative genetics: biological meaning of the parameters. Evolution 46, 540–550 (doi:10.2307/2409870) [DOI] [PubMed] [Google Scholar]
- Kramer S. J., Kalish F.1984Regulation of the coprora allata in the black mutant of Manduca sexta. J. Insect Physiol. 30, 311–316 (doi:10.1016/0022-1910(84)90132-X) [Google Scholar]
- Kruuk L. E. B., Slate J., Wilson A. J.2008New answers for old questions: the evolutionary quantitative genetics of wild animal populations. Annu. Rev. Ecol. Evol. Syst. 39, 525–548 (doi:10.1146/annurev.ecolsys.39.110707.173542) [Google Scholar]
- Lande R.1980Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- Lindenfors P., Gittleman J. L., Jones K. E.2007Sexual size dimorphism in mammals. In Sex, size and gender roles: evolutionary studies of sexual size dimorphism (eds Fairbairn D. J., Blanckenhorn W. U., Székely T.), pp. 16–26 New York, NY: Oxford University Press [Google Scholar]
- Lynch M., Walsh B.1998Genetics and analysis of quantitative traits Sunderland, MA: Sinauer Associates [Google Scholar]
- Mirth C. K., Riddiford L. M.2007Size assessment and growth control: how adult size is determined in insects. BioEssays 29, 344–355 (doi:10.1002/bies.20552) [DOI] [PubMed] [Google Scholar]
- Nijhout H. F., Davidowitz G.2009The developmental-physiological basis of phenotypic plasticity. In Phenotypic plasticity of insects: mechanisms and consequences (eds Whitman D. W., Ananthakrishnan T. N.), pp. 589–608 Enfield, NH: Science Publishers [Google Scholar]
- Nijhout H. F., Davidowitz G., Roff D. A.2006A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 5, 16.11–16.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhout H. F., Roff D. A., Davidowitz G.2010Conflicting processes in the evolution of body size and development time. Phil. Trans. R. Soc. B 365, 567–575 (doi:10.1098/rstb.2009.0249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth A. P., Goodman W. G.1995Juvenile hormone regulation of hemolymph juvenile hormone binding protein in the black strain of the tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 30, 165–176 (doi:10.1002/arch.940300207) [Google Scholar]
- Poissant J., Coltman D. W.2009The ontogeny of cross-sex genetic correlations: an analysis of patterns. J. Evol. Biol. 22, 2558–2562 (doi:10.1111/j.1420-9101.2009.01862.x) [DOI] [PubMed] [Google Scholar]
- Poissant J., Wilson A. J., Coltman D. W.2010Sex-specific genetic variance and the evolution of sexual dimorphism: a systematic review of cross-sex genetic correlations. Evolution 64, 97–107 (doi:10.1111/j.1558-5646.2009.00793.x) [DOI] [PubMed] [Google Scholar]
- Raguso R. A., Willis M. A.2002Synergy between visual and olfactory cues in nectar feeding by naïve hawkmoths. Anim. Behav. 64, 685–695 (doi:10.1006/anbe.2002.4010) [Google Scholar]
- Reeve J. P., Fairbairn D. J.1996Sexual size dimorphism as a correlated response to selection on body size: an empirical test of the quantitative genetic model. Evolution 50, 1927–1938 (doi:10.2307/2410751) [DOI] [PubMed] [Google Scholar]
- Roff D. A.1997Evolutionary quantitative genetics New York, NY: Chapman and Hall [Google Scholar]
- Roff D. A., Preziosi R.1994The estimation of the genetic correlation: the use of the jackknife. Heredity 73, 544–548 (doi:10.1038/hdy.1994.153) [Google Scholar]
- Safranek L., Riddiford L. M.1975The biology of the black larval mutant of the tobacco hornworm, Manduca sexta. J. Insect Physiol. 21, 1931–1938 (doi:10.1016/0022-1910(75)90225-5) [Google Scholar]
- Shingleton A. W., Frankino W. A., Flatt T., Nijhout H. F., Emlen D. J.2007Size and shape: the regulation of static allometry in insects. BioEssays 29, 536–548 (doi:10.1002/bies.20584) [DOI] [PubMed] [Google Scholar]
- Shingleton A. W., Mirth C. K., Bates P. W.2008Developmental model of static allometry in holometabolous insects. Proc. R. Soc. B 275, 1875–1885 (doi:10.1098/rspb.2008.0227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillwell R. C., Blanckenhorn W. U., Teder T., Davidowitz G., Fox C. W.2010Sex differences in phenotypic plasticity of body size affect variation in sexual size dimorphism in insects: from physiology to evolution. Annu. Rev. Entomol. 55, 227–245 (doi:10.1146/annurev-ento-112408-085500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windig J. J.1997The calculation and significance testing of genetic correlations across environments. J. Evol. Biol. 10, 853–874 (doi:10.1007/s000360050058) [Google Scholar]