Abstract
Collagen fibers affect metastasis in two opposing ways, by supporting invasive cells but also generating a barrier to invasion. We hypothesized that these functions might be performed by different isoforms of type I collagen. Carcinomas are reported to contain α1(I)3 homotrimers, a type I collagen isoform normally not present in healthy tissues, but the role of the homotrimers in cancer pathophysiology is unclear. In this study, we found that these homotrimers were resistant to all collagenolytic matrix metalloproteinases (MMPs). MMPs are massively produced and utilized by cancer cells and cancer-associated fibroblasts for degrading stromal collagen at the leading edge of tumor invasion. The MMP-resistant homotrimers were produced by all invasive cancer cell lines tested, both in culture and in tumor xenografts, but they were not produced by cancer-associated fibroblasts, thereby comprising a specialized fraction of tumor collagen. We observed the homotrimer fibers to be resistant to pericellular degradation, even upon stimulation of the cells with pro-inflammatory cytokines. Further, we confirmed an enhanced proliferation and migration of invasive cancer cells on the surface of homotrimeric vs. normal (heterotrimeric) type I collagen fibers. In summary, our findings suggest that invasive cancer cells may utilize homotrimers for building MMP-resistant invasion paths, supporting local proliferation and directed migration of the cells while surrounding normal stromal collagen is cleaved. Because the homotrimers are universally secreted by cancer cells and deposited as insoluble, MMP-resistant fibers, they offer an appealing target for cancer diagnostics and therapy.
Keywords: collagen homotrimers, MMP, collagen degradation, cell-matrix interactions, collagenases
Introduction
Overexpression of matrix metalloproteinases (MMPs) is a hallmark of invasive cancers (1, 2). This enzyme family has multiple functions, including cleaving various extracellular matrix components (3, 4). MMPs capable of cleaving type I collagen triple helices, the main matrix component in many tissues, are collagenases (MMP-1, MMP-8, MMP-13, and MMP-14) and gelatinase A (MMP-2) (5). Membrane-bound MMP-14 may play a particularly important role, e.g., MMP-14 knockout mice have severe collagen turnover deficiency (6). One of the critical functions of collagenolytic MMPs in cancer is clearing an invasion path through the barrier of type I collagen fibers in stroma and blood vessel walls.
However, collagen fibers not only hinder cancer invasion; they also promote the invasion by inducing the epithelial-mesenchymal transition (EMT) and supporting the invasiveness, proliferation, and migration of cancer cells (7, 8). Consistent with this dual role, invasive cancer cells and cells recruited into tumors produce both an increased amount of MMPs and an increased amount of collagen (8, 9). Remarkably, the invasion-supporting function may be performed, at least in part, by a type I collagen isoform normally not present in healthy tissues.
The normal isoform of type I collagen is a heterotrimer of two α1(I) and one α2(I) chains (α12α2). A fetal, homotrimeric isoform (α13) with three α1(I) chains was found in carcinomas (10-14) and in cultures of chemically transformed (15, 16) and cancer (17-21) cells. It was suggested that the homotrimers may enhance proliferation (22) and migration (23) of cancer cells, but their role in cancer pathophysiology was not established.
In the present study, we hypothesized that the homotrimers might be selectively produced by cancer and not surrounding cells, forming MMP-resistant roadways for tissue invasion and providing the necessary supporting function of collagen without the inhibitory barrier effect. We took a cue from an observation that homotrimers refolded in vitro from denatured α1(I) chains were resistant to MMP-1 and MMP-8 (24); although the refolding could result in improper chain register, disrupting the normal collagenase cleavage site. Testing of our hypothesis revealed that naturally produced homotrimers were indeed resistant to cleavage by all collagenolytic MMPs (1, 2, 8, 13, and 14) and to degradation by fibroblasts and cancer cells. In culture, the homotrimers comprised 15 to 40% of type I collagen secreted by invasive melanoma, adenocarcinoma, fibrosarcoma, and neuroblastoma cells, but they were not produced by normal fibroblasts. In xenograft tumors, the homotrimers comprised about 50% of type I collagen produced by the same human cancer cells, but no homotrimers were produced by mouse cells recruited into the tumors. Finally, we confirmed faster proliferation and migration of cancer cells on matrix reconstituted from the homotrimers compared to heterotrimers.
Materials and Methods
Cell culture
CRL-2127 fibroblasts and HT-1080 fibrosarcoma cells were purchased from the American Type Culture Collection (ATCC); PacMet cells were generously supplied by Dr. Linda A. deGraffenried, University of Texas at Austin. All other cells were obtained from the National Cancer Institute Drug Screen and characterized by analysis of short tandem repeats (Suppl. Table S1). Cells were cultured at 37 °C, 5% CO2 in Dulbecco’s modified Eagle’s medium with 2 mM GlutaMAX™ (DMEM, Invitrogen) and 10% fetal bovine serum (FBS, Gemini Bioproducts). After 70-90% confluence, the cells were incubated for 24 h in DMEM/GlutaMAX™ with 0.1% FBS. The harvested medium was buffered with 100 mM Tris-HCl, pH 7.4, protected with protease inhibitors, and used for purification of collagen as described in (25). In some experiments, collagen synthesis was stimulated with 50 μg/ml ascorbate or 5 ng/ml TGF-β1 (PeproTech). The cells were released from flask surface by 0.05% trypsin-EDTA (Invitrogen) and counted.
Type I collagen purification, labeling, and characterization
Mouse heterotrimers and homotrimers were extracted from tail and spinal tendons of wild type and homozygous oim mice, respectively, with 0.5 M acetic acid (acid-soluble) or with 0.1 mg/ml pepsin in 0.5 M acetic acid (pepsin-treated) and purified by selective precipitation with 0.7 M NaCl (26). Human heterotrimers were isolated from cultured CRL-2127 fibroblasts (25) and homotrimers were isolated from cultured fibroblasts with two nonfunctional COL1A2 alleles (27) generously provided by Dr. Peter Byers, University of Washington. Collagen concentrations were measured by circular dichroism (CD). Collagen was labeled with amino-reactive Alexa Fluor 488 (AF488) carboxylic acid succinimidyl ester (Invitrogen), DyLight 549 or 649 NHS-esters (Pierce), or Cy5 NHS-ester (GE Healthcare) (25). The labeling efficiency was adjusted to 1 dye per 3-10 collagen molecules. Labeled collagen was characterized by gel electrophoresis on precast 3-8% Tris-Acetate mini gels (Invitrogen); the gels were scanned on an FLA5000 fluorescence scanner (Fuji Medical Systems) and analyzed with Multi-Gauge software supplied with the scanner. The identity of polypeptides migrating at the α(I) and α2(I) collagen chain positions was confirmed by CNBr digestion and analysis of CNBr-peptides on precast 12% Tris-Gly mini gels (Invitrogen).
To quantify collagen secretion, AF488-labeled MMP-1-fragments of type I mouse-tail-tendon collagen (6.6 ng/ml) were added to a media aliquot before collagen purification, as an internal standard. After the purification, the ratio of full-length α1(I) chains to ¾-MMP-1-fragments of α1(I) chains was measured by Cy5-labeling and gel electrophoresis.The initial concentration of secreted collagen in cell culture media was determined by comparing the measured α1(I) : ¾-α1(I) ratio to mock experiments, in which the ¾-fragments were mixed in a known proportion with purified procollagen.
Collagen cleavage with soluble MMPs
Recombinant human proMMP-1 and proMMP-13 were prepared, activated, and purified as described in (28). Active human neutrophil MMP-8 and recombinant human MMP-2 were purchased from EMD Biosciences. Binary mixtures of human or mouse type I collagen homotrimers and heterotrimers, one component labeled with AF488 and the other with Cy5, were prepared in 50 mM Tris–HCl (pH 7.5), 0.15 M NaCl, 5 mM CaCl2, 0.05 % Brij 35 (TNC buffer) and incubated with MMP-1 (2 nM), MMP-2 (75 nM), MMP-8 (~50 nM), or MMP-13 (10 nM). Aliquots taken at different time intervals were mixed with a lithium dodecyl sulfate (LDS) gel-loading buffer (Invitrogen) and 20 mM EDTA, denatured and analyzed by gel electrophoresis.
Homotrimer assays
AF488-labeled heterotrimers were mixed 1:1 with Cy5-labeled unknown samples or with Cy5-labeled calibration samples with known homotrimer fractions. The mixtures (0.05-0.1 mg/ml) were incubated with 2 nM activated MMP-1 at 25°C. Aliquots collected after different time intervals were analyzed by gel electrophoresis. To disrupt pepsin-resistant intermolecular cross-links, tissue-derived samples were treated with 0.05 mg/ml pronase (EMD Biosciences) at 4°C before the electrophoresis. Comparison of the fraction of uncleaved, Cy5-labeled α1(I) chains in the unknown sample with the calibration mixtures allowed detection of 1% homotrimers and measurement of the homotrimer fraction with relative accuracy better than 10%. The best accuracy was achieved at 90-95% cleavage of the AF488-labeled heterotrimers. In addition, we measured the α1(I)/α2(I) chain ratio in initial mixtures and as a function of the incubation time.
Pericellular degradation of fibrillar collagen
Fibrillar matrix was reconstituted from acid-soluble homotrimeric and heterotrimeric mouse-tail-tendon type I collagens. Ice-cold solutions of DyLight-649-labeled homo- and heterotrimers in 2 mM HCl (0.4 mg/ml) were mixed 9:1 with ice-cold 10x-PBS and placed into wells of pre-cooled multi-well, chambered coverslips (Invitrogen). Collagen fibril formation was induced by incubating the coverslips at 32°C for at least 1 hour followed by overnight incubation at 37°C. The resulting fibril gels were dried into films under a flow of air in a cell culture hood. The films were sterilized with 70% ethanol, and imaged in the FLA5000 fluorescence scanner at 10-25 μm resolution. Wild type and MMP-14-knock-out mouse fibroblasts (6) were stimulated with 1 nM IL-1β (PeproTech) and 10 nM TNF-α (PeproTech) for 24 hours as described in (29). The stimulated cells were seeded in the middle of the reconstituted collagen films (10,000-20,000 cells/well) and allowed to attach for 6-16 h. The media was then replaced with DMEM / 0.5% FBS / 1 nM IL-1β / 10 nM TNF-α. After 3-4 day incubation at 37°C, 5% CO2, the films were fixed with 2% formaldehyde (1 h at 37°C), labeled with DAPI, re-examined in the FLA5000 scanner, and imaged in a wide field fluorescence microscope (Olympus BX-51) or a confocal microscope (Zeiss LSM 510 Meta) at different resolutions.
Cell adhesion, proliferation and migration
Heterotrimer and homotrimer films were prepared from pepsin-treated mouse-tail-tendon collagen in 96-well plates as described above. In cell adhesion assay, 100,000 cells/well were seeded on these films and incubated for 30 min at 37°C. Non-adherent cells were washed out with media. The remaining cells were labeled with calcein (Invitrogen) and total fluorescence intensity of each film was measured in the FLA5000 fluorescence scanner. The number of adherent cells was determined based on calibration curves for films with known cell density. The proliferation assay was essentially similar, but 100-1000 cells/well were seeded, allowed to adhere and proliferate for 4 days. Cell migration was measured using Platypus 96-well plate (Platypus). A central circle in each collagen film was blocked with a stopper and cells were seeded outside the stopper. Once the cells adhered, the stopper was removed and cells were allowed to move into the central circle for 1-2 days. The cells were then labeled with calcein, the outside area was masked and the fluorescence intensity from the cells, which migrated into the central circle, was measured.
Xenograft tumors
Tumors generated in athymic nude mice (nu/nu NCr) by injection of LOX-IMVI melanoma, PC-3 prostate cancer, and MDA-MB-231 breast cancer cells (at least 3 tumors for each cell line) were obtained from Dr. Melinda Hollingshead, National Cancer Institute. The LOX-IMVI and MDA-MB-231 tumors were collected from the first passage in mice; PC-3 tumors were collected from the second passage. The tumors were minced and collagen was extracted in 0.1 mg/ml pepsin, 0.5 M acetic acid, 0.5% Brij 35 at 4°C for 24-48h. Extraction was repeated 4-5 times until all collagen was solubilized. Collagen was purified by several rounds of precipitation with 0.7 M NaCl and examined by gel electrophoresis after Cy5 labeling. Thermal denaturation thermograms in 0.2 M Na-phosphate, 0.5 M glycerol (pH 7.4) were measured by differential scanning calorimetry (DSC) in a Nano III calorimeter (TA Instruments) or by CD in a J810 spectropolarimeter (Jasco Inc.) with a thermoelectric device (30). A sample of collagen from each tumor was analyzed with the MMP-1 cleavage assay as described above. The remaining collagen was heated in the TNC buffer to 39°C for 10-20 min, cooled to 20°C and treated with 2.5 mg/ml trypsin, 2.5 mg/ml chymotrypsin for 10 min. The reaction was stopped by adding 0.5 M acetic acid; and undigested collagen was re-precipitated with 0.7 M NaCl. This procedure resulted in complete digestion of the less stable collagen triple helices produced by mouse cells while ~50 % of collagen produced by human cancer cells remained intact. The resulting human collagen was reanalyzed by gel electrophoresis, MMP-1 digestion, and CD.
Results
MMP resistance
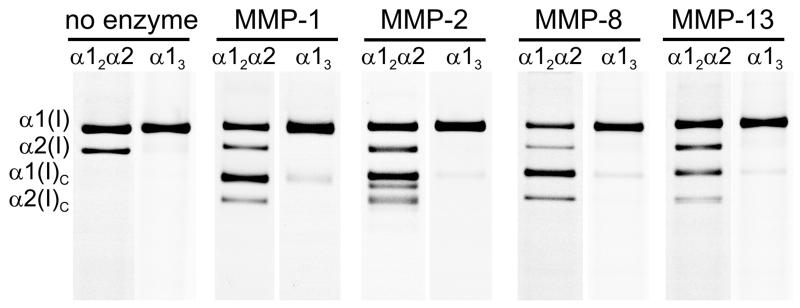
We tested the MMP resistance of α1(I) homotrimers produced by human fibroblasts with nonfunctional COL1A2 alleles (27) as well as α1(I) homotrimers from tail tendons of homozygous oim mice with nonfunctional α2(I) chains (31). We mixed fluorescently labeled homotrimers with the corresponding human or mouse heterotrimers labeled with a different dye. Each mixture was then processed with recombinant human MMP-1, MMP-2, MMP-8, or MMP-13 at temperatures from 20 to 35°C. Gel electrophoresis of aliquots collected at different time revealed cleavage of type I homo- and heterotrimers at the same sites, producing the expected 3/4 and 1/4 fragments (Fig. 1, Suppl. Fig. S1). Additional fragments were observed only upon cleavage with MMP-2.
Figure 1.
Collagen cleavage by interstitial MMPs in binary homo/heterotrimer mixtures. Each pair of images shows the same gel lane in two fluorescent colors, corresponding to hetero- and homotrimer labeling; α1(I)C and α2(I)C are the 3/4-fragments produced by MMP cleavage at the expected site after Gly-775 (see Suppl. Fig. S1). Only 1-5% homotrimers were cleaved (faint α1(I)C bands in α13 lanes) at the incubation time with ~50% heterotrimer cleavage. The same results were observed for human collagen (shown for MMP-1 and MMP-2) and mouse collagen (shown for MMP-8 and MMP-13).
The homotrimer cleavage rates by all MMPs at all temperatures were more than 5-10 times slower than heterotrimer cleavage rates, both for human and mouse collagens. The cleavage kinetics of naturally produced homotrimers by MMP-1 was consistent with the homotrimers refolded in vitro (24). Confocal imaging showed that the reconstituted homotrimer fibers were also much more resistant to cleavage at 37°C.
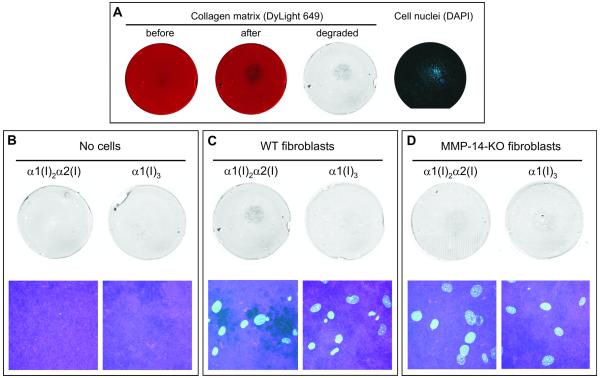
Next, we compared pericellular cleavage of collagen matrix by fibroblasts from wild type (WT) and MMP-14 knockout (KO) mice (6). The cells were seeded on 2 μm-thick, fluorescently labeled matrix reconstituted from hetero- or homotrimeric type I collagen fibers. Cells were stimulated with TNF-α and IL-1β to speed up matrix degradation (29). After 3-4 day culturing, matrix and cells were fixed and imaged (Fig. 2). WT cells made holes in type I heterotrimer matrix, clearly visible both at low and high resolution. The degradation of type I heterotrimer films by KO cells was strongly suppressed (6). We observed no detectable pericellular degradation of type I homotrimer films by either WT or KO cells. Normal appearance of nuclei suggested that the cells were viable. Thus, type I homotrimer fibers were resistant to pericellular degradation in general and to MMP-14, in particular. Similarly, we found that homotrimer fibers were resistant to pericellular cleavage by HT-1080 fibrosarcoma cells with and without the stimulation with TNF-α and IL-1β as well as to pericellular cleavage by LOX-IMVI melanoma cells (Suppl. Fig. S2).
Figure 2.
Pericellular degradation of collagen matrix by MMP-14. A, Matrix degradation by mouse fibroblasts stimulated with TNFα̣/ IL-1β. DyLight-649-labeled collagen matrix (red) was imaged before and after 3 day incubation with the fibroblasts seeded in the center of the film. Cell nuclei were imaged after DAPI labeling. The grayscale image of matrix degradation was obtained by subtraction of the matrix fluorescence intensity after 3 day incubation with the cells from the matrix fluorescence intensity before the incubation; darker areas represent larger loss of fluorescence. The loss of fluorescence was caused by mechanically damaging the film with a pipette tip (e.g., black spot in the lower left quadrant), bleaching of the fluorescence (overall background, see panel B), and matrix degradation by cells (round area in the middle, surrounding cell nuclei). B-D, Fluorescence bleaching without cells (B) and matrix degradation by wild-type (C) and MMP-14-knockout (D) fibroblasts. Low resolution difference images of matrix degradation (top) were obtained as described in panel A. High-resolution images are projections of confocal stacks within the areas populated by cells (bottom). The cyan channel shows DAPI-labeled cell nuclei and magenta channel shows DyLight-649-labeled collagen. The dark areas in the confocal image of the heterotrimer matrix with WT fibroblasts (C, lower left image) are holes cut in the matrix by the cells. No holes were observed in the homotrimer matrix (C, lower right image) or with MMP-14-knockout fibroblasts (D).
Homotrimer synthesis by cancer cells in culture
Type I collagen homotrimers found in carcinomas and carcinoma cell cultures could be selectively synthesized by cancer cells. However, they could also be products of cancer associated fibroblasts and/or byproducts of residual homotrimer synthesis by all cells and selective heterotrimer degradation by collagenases massively produced within tumors. To clarify the homotrimer origin, we analyzed procollagen secreted into cell culture medium by normal dermal fibroblasts (CRL-2127) and nine cell lines from different types of cancer (Table 1).
Table 1.
Secretion of type I collagen by normal and cancer cells
| Cell line | Cell origin | Collagen (fg) /cell† | % Homotrimers † | |
|---|---|---|---|---|
| MMP-1 assay | α1/α2 assay | |||
| CRL-2127 | Foreskin, normal fibroblast | 7.5 | 0 | 0 |
| LOX-IMVI | Melanoma | 2.7 | 25 | 30 |
| MCF-7 | Mammary adenocarcinoma | 0.6 | 27 | 25 |
| MDA-mb-231 | Mammary adenocarcinoma | 1.1 | 37 | 32 |
| MDA-mb-435 | Mammary adenocarcinoma | 0.3 | n.d. | 20 |
| LNCaP | Prostate adenocarcinoma | 0.5 | 25 | 30 |
| PacMet | Prostate adenocarcinoma | 0.4 | 17 | 25 |
| PC-3 | Prostate adenocarcinoma | 1.1 | 25 | 35 |
| HT-1080 | Fibrosarcoma | 1.7 | 37 | 28 |
| SY5Y | Neuroblastoma | 1.6 | 14 | 25 |
The collagen yield and homotrimer fraction varied by up to a factor of 2 from experiment to experiment. Hence, the average values reported in this table are intended to serve only as estimates.
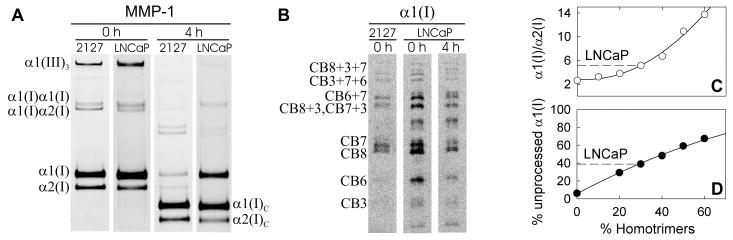
Within 24 h, CRL-2127 fibroblasts secreted ~ 7 fg collagen per cell, in which no homotrimers were detected. Under the same conditions, cancer cells secreted 0.3-3 fg/cell type I collagen, of which 15-40% were α1(I) homotrimers. Figure 3 demonstrates the presence of an MMP-1-resistant, homotrimeric type I collagen in media from LNCaP cells (A,B) and illustrates assays for the homotrimer fraction based on the α1(I)/α2(I) chain ratio (C) and on the fraction of uncleaved α1(I) chains after 4-8h incubation with MMP-1 (D). The homotrimers were not byproducts of selective heterotrimer degradation by collagenases since such degradation was negligible: (a) We observed no change in the homo/heterotrimer ratio when a mixture of fluorescently labeled procollagens was prepared in a cell culture medium and incubated with cells for 24 h. (Suppl. Fig. S3) (b) We did not detect any collagen degradation upon mixing collagen solution 1:1 with the conditioned medium and only minimal degradation when MMPs in the conditioned medium were activated at 37°C for 1h with 1 mM p-aminophenylmercuric acetate. (Suppl. Fig. S3)
Figure 3.
Measurement of the homotrimer fraction in type I collagen secreted into cell culture media. A, Gel electrophoresis of Cy5-labeled-LNCaP-collagen mixed with AF488-labeled-CRL-2127-collagen before and after 4h incubation with activated MMP-1. Each pair of images represents the same gel lane scanned for AF488 (2127) and Cy5 (LNCaP) fluorescence, respectively. B, Gel electrophoresis of peptides obtained by CNBr cleavage of α1(I) bands cut out from the gels shown in panel A. The 2127 peptide pattern corresponds to the expected cleavage products of α1(I) chains (e.g., CB7 is the CNBr peptide 7 of the α1(I) chain and CB3+7+6 is the partial cleavage product with intact bonds between CB7 and adjacent CB3 and CB6). The LNCaP peptide pattern shows only minor (<10%) contamination of the α1(I) gel band with chain(s) of unknown origin (the band above CB7). C, The α1(I)/α2(I) ratios of fluorescence intensities of the gel bands for LNCaP collagen (dashed line) and homo/heterotrimer mixtures with known composition (open circles and solid regression curve). D, The fraction of unprocessed α1(I) chains after 4h cleavage with MMP-1 for LNCaP collagen (dashed line) and homo/heterotrimer mixtures with known composition (filled circles and solid regression curve). The x-coordinates of the intersections between the dashed and solid lines in C and D give the type I homotrimer fraction in LNCaP collagen (~30% in both assays).
We also tested the effects of factors known to regulate collagen production. In particular, ascorbic acid (50 μg/ml) increased the total collagen yield from CRL-2127 fibroblasts 200-300 times, but it did not induce the homotrimer synthesis and it had almost no effect on other cells. TGF-β1 (5 ng/ml) increased the collagen yield from CRL-2127 fibroblasts up to 10-15 times, also without inducing the homotrimer synthesis. Exogenous TGF-β1 had more complex, condition-dependent effects on cancer cells, in some cases increasing (but never decreasing) the homotrimer synthesis.
Homotrimer synthesis by cancer cells in vivo
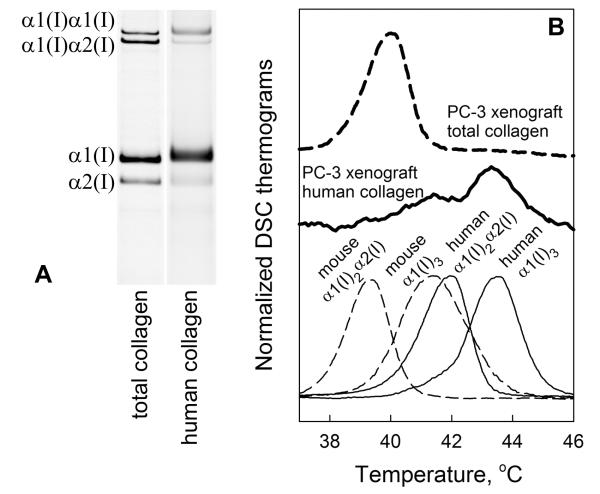
For in vivo testing of type I homotrimer synthesis we selected xenograft tumors, in which we could distinguish type I collagen produced by host and engrafted cells based on different denaturation temperature (Tm) of mouse and human collagens. We purified type I collagen from tumors produced in athymic nude mice by LOX-IMVI, PC-3, and MDA-mb-231 cells with pepsin digestion and selective salt fractionation. Analysis by DSC and CD (30), gel electrophoresis, and MMP-1 digestion revealed that normal heterotrimers of murine origin comprised >95% of total type I tumor collagen (Fig. 4 and Suppl. Fig. S4).
Figure 4.
Collagen synthesis in xenograft tumors. A, Gel electrophoresis of total collagen and human collagen fractions in PC-3 xenograft tumor (note slightly slower migration of human collagen). B, DSC denaturation thermogram of total xenograft collagen (bold-dashed); CD denaturation thermogram of human xenograft collagen (bold); and DSC thermograms of mouse hetero- and homotrimers from spinal tendons (thin-dashed) and human hetero- and homotrimers produced in cell culture (thin). DSC thermograms of xenograft collagen are compared with mouse collagen from different tissues in Suppl. Fig. S4.
To separate a small fraction of human collagen produced by cancer cells from the murine collagen, we briefly incubated collagen solution above the Tm of heterotrimeric mouse collagen but below the Tm of human collagen, cooled the solution to room temperature, degraded denatured mouse collagen with a trypsin/chymotrypsin mixture, and re-precipitated intact collagen, which comprised 1-2% of the initial sample. The CD denaturation thermogram of this collagen (Fig. 4B, bold dashed line) revealed two denaturation peaks; the more stable fraction consistent with human homotrimers and less stable fraction consistent with human heterotrimers and/or murine homotrimers. From relative intensities of the two peaks, we measured the fraction of human homotrimers (30). Comparison of this fraction with the α1(I)/α2(I) chain ratio in gel electrophoresis suggested that the re-precipitated collagen was predominantly human, i.e., virtually all mouse collagen appeared to be degraded. This conclusion was supported by differences in electrophoretic mobilities of the α1(I) and α2(I) chains in the initial tumor sample and re-precipitated collagen (Fig. 4A). Comparison with calibration mixtures of known composition (after the same treatment) allowed us to correct for partial degradation of human heterotrimers and estimate the tumor collagen composition.
Thus, we deduced that recruited (cancer-associated) mouse cells produced 98-99% of tumor type I collagen, all of which was heterotrimeric. In contrast, transplanted human cancer cells synthesized 1-2% of tumor type I collagen, ~50% of which was homotrimeric.
Proliferation and migration of cancer cells on homotrimer matrix
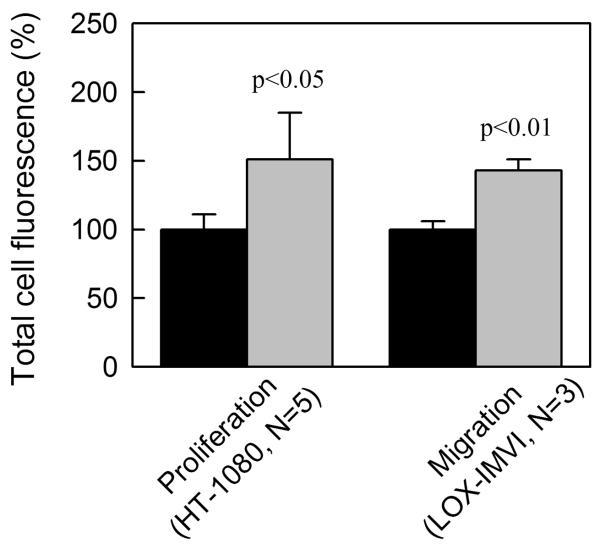
Previous studies revealed faster proliferation (22) and migration (23) of 8701-BC breast carcinoma cells on homotrimeric type I collagen deposited from 0.5 M acetic acid by incubation at 37°C or air drying. To compare cancer cell interactions with heterotrimer and homotrimer collagen fibers prepared under physiological conditions, we reconstituted thin (1-2 μm), dense films of mouse-tail-tendon type I collagen fibers by in vitro fibrillogenesis. Consistent with the results for 8701-BC cells on acid-deposited human collagen (22, 23, 32), we observed no significant difference in cell adhesion, but ~50% faster proliferation of HT-1080 cells and ~40% faster migration of LOX-IMVI cells on the homotrimer matrix (Fig. 5 and Suppl. Fig. S5).
Figure 5.
Proliferation and migration of cancer cells on heterotrimer (black) and homotrimer (grey) matrix. Cells were seeded on dense, thin films reconstituted by in vitro fibrillogenesis from wild type (heterotrimers) and oim (homotrimers) mouse-tail-tendon type I collagen. The number of cells was evaluated by calcein fluorescence after 4 day proliferation within whole matrix area or 1-2 day migration into an initially blocked area (Suppl. Fig. S5). The results were normalized based on the average fluorescence of cells on heterotrimer matrix.
Discussion
Collagen fibers in stroma and vasculature as well as fibers produced by cancer-associated (recruited) fibroblasts form an invasion barrier. At the same time, collagen fibers are essential for maintaining the invasive phenotype and supporting migration and proliferation of cancer cells. Massive production of both collagen and MMPs that degrade collagen, mostly by cancer-associated fibroblasts, may be one solution to this dilemma. However, the present study suggests that invasive cells may benefit even more from producing fibers of MMP-resistant homotrimeric type I collagen. Not only are the homotrimers resistant to all collagenolytic MMPs in solution (MMP-1, 2, 8, 13; Fig. 1), but the homotrimer matrix is resistant to cleavage by fibroblasts (Fig. 2) and cancer cells (Supp. Fig. S2), even when the cells are stimulated with proinflammatory cytokines.
Because the homotrimer fibers appear to be produced only by cancer cells and not by cancer-associated fibroblasts (Table 1 and Fig. 3, 4), they comprise a small fraction of tumor collagen. As a result, cancer cells may utilize these fibers as MMP-resistant roadways for invasion rather than as building materials for the tumor stroma. Our data suggest that these collagenase-resistant fiber roadways may provide the necessary support for proliferation and migration of the cancer cells without forming invasion barriers (Suppl. Fig. S6). They may also promote better organization and migration of all tumor cells and more directed and efficient degradation of surrounding stroma.
In addition to MMP resistance, type I collagen homotrimer fibers have distinct mechanical properties (33, 34). Matrix mechanics plays an important role in malignancy, significantly affecting the behavior of cancer cells (35-38). For instance, higher rigidity of the homotrimer fibers (34) may contribute to faster proliferation and migration of cancer cells. Potential differences in binding of proteoglycans, cytokines, and other matrix molecules to homotrimer fibers may also affect cancer microenvironment.
Note that collagenase-resistant fibers can still be degraded by other enzymes that cleave non-helical terminal peptides. Such cleavage is likely involved in normalizing collagen turnover in Col1a1r/r and oim mice; which make collagenase-resistant type I collagen but exhibit only mild localized fibrosis (39-42). (Type I collagen has an altered MMP cleavage site in Col1a1r/r and is homotrimeric in oim mice). Alternative collagen cleavage does not prevent severe, generalized collagen turnover deficiency in MMP-14-knockout mice (6); although this may be related to other functions of the multifunctional (43) MMP-14. Alternative cleavage may also be less important in cancer progression.
Consistent with our hypothesis for the role of type I collagen homotrimers in cancer invasion, the same cancer cells seem to produce a higher fraction of the homotrimers in vivo (~50%, Fig. 4) than in vitro (25-35%, Table 1). The higher homotrimer content of cancer cell derived collagen in xenograft tumors may result from selective degradation of the heterotrimers by collagenases. However, it may also be caused by selective proliferation of cancer cell subpopulations producing more homotrimers. Indeed, a several fold reduction in the relative amount of the α2(I) chain mRNA compared to the α1(I) chain mRNA was observed after several cycles of selection for more aggressive TC-1 cells in C57BL/6 mice, as reported in the Gene Expression Omnibus database, NCBI, accession # GSE2774 (44).
What are the factors that enable cancer cells to produce homotrimeric type I collagen? These cells are exposed to the same environment as cancer-associated fibroblasts, which do not make the homotrimers. Thus, the answer likely lies within the cells themselves. One possibility is insufficient expression of the α2(I) chain, e.g., due to methylation of the α2(I) gene (45). However, similar homotrimer synthesis by different cancer cells (Table I) would then mean similar α2(I) chain expression deficiency. Such similarity between different cancers seems unlikely.
Another clue to answering this question may be contained in comparing different types of cells producing the homotrimers. From literature analysis, we found no convincing evidence for the homotrimer production by cells normally responsible for collagen synthesis, except for cases with deficient α2(I) chain synthesis caused by rare mutations. In our own measurements, we also observed no detectable homotrimers in (i) fibroblast and osteoblast cultures (human and murine); (ii) murine skin, tendons, and bone; and (iii) normal and fibrotic human skin. In contrast, the homotrimer synthesis by embryonic cells (46), dedifferentiated cells (47), non-osteogenic bone marrow cells (48), chemically transformed cells (15, 16), cancer cells (17-21), and stressed mesangial cells (49) has been well documented. These observations suggest that mature collagen-producing cells may have a mechanism for preventing the α1(I) homotrimer formation when a sufficient number of the α2(I) chains is synthesized, e.g., a specialized chaperone that promotes association and folding of two α1(I) with one α2(I) C-propeptide chains. This mechanism may be absent in cells that normally produce little or no type I collagen, in which the corresponding chaperone may not be expressed. This mechanism may also be absent or not fully functional in fetal cells.
Regardless of the underlying mechanism, homotrimeric type I collagen appears to be produced only in fetal or pathological tissues. This property may be utilized for diagnostic and therapeutic targeting of cancer and other pathologies involving the homotrimer synthesis, e.g., for visualizing peripheral areas invaded by cancer cells during surgery. Indeed, insoluble, collagenase-resistant homotrimer fibers may present an ideal target, provided that a molecule that selectively binds to the homotrimers but not heterotrimers can be designed. This task may be challenging since the homotrimers do not contain unique peptide sequences. However, some triple helix regions are much less stable within the homotrimers than within the heterotrimers (26). Targeting these regions by molecules that recognize unfolded but not folded chains may present one possible solution.
Supplementary Material
Acknowledgments
We thank Drs. Peter H. Byers, Linda A. deGraffenried, Melinda Hollingshead, Daniel McBride, James Pace, and Ulrike Schwarze for generously providing tissues and cells used for this study. Confocal microscopy was performed at the Microscopy & Imaging Core of NICHD with the assistance of Dr. Vincent Schram.
Grant Support This work was supported in part by the Intramural Research Programs of NICHD (Leikin) and NIDCR (Holmbeck), NIH, Wellcome Trust Programme Grant 075473 (Nagase), and NIH/NIDDK Grant DK069522 (Phillips).
Footnotes
Conflicts of Interest The authors declare no competing commercial interest with the work presented here.
REFERNCES
- 1.Liotta LA, Stetler-Stevenson WG. Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 1991;51:5054s–9s. [PubMed] [Google Scholar]
- 2.Mignatti P, Rifkin DB. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993;73:161–95. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- 3.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 4.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 5.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Holmbeck K, Bianco P, Caterina J, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 7.Grzesiak JJ, Ho JC, Moossa AR, Bouvet M. The integrin-extracellular matrix axis in pancreatic cancer. Pancreas. 2007;35:293–301. doi: 10.1097/mpa.0b013e31811f4526. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 9.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 10.Pucci-Minafra I, Andriolo M, Basirico L, et al. Absence of regular alpha2(I) collagen chains in colon carcinoma biopsy fragments. Carcinogenesis. 1998;19:575–84. doi: 10.1093/carcin/19.4.575. [DOI] [PubMed] [Google Scholar]
- 11.Shapiro FD, Eyre DR. Collagen polymorphism in extracellular matrix of human osteosarcoma. J Natl Cancer Inst. 1982;69:1009–16. [PubMed] [Google Scholar]
- 12.Yamagata S, Yamagata T. FBJ virus-induced osteosarcoma contains type I, type I trimer, type III as well as type V collagens. J Biochem. 1984;96:17–26. doi: 10.1093/oxfordjournals.jbchem.a134809. [DOI] [PubMed] [Google Scholar]
- 13.Moro L, Smith BD. Identification of collagen alpha1(I) trimer and normal type I collagen in a polyoma virus-induced mouse tumor. Arch Biochem Biophys. 1977;182:33–41. doi: 10.1016/0003-9861(77)90280-6. [DOI] [PubMed] [Google Scholar]
- 14.Asokan R, Puvanakrishnan R, Ravichandran LV, Kokila V, Reddy GK, Dhar SC. Purification and characterization of collagens from rat fibrosarcoma induced by 3-methylcholanthrene. Mol Cell Biochem. 1993;121:99–107. doi: 10.1007/BF00925968. [DOI] [PubMed] [Google Scholar]
- 15.Marsilio E, Sobel ME, Smith BD. Absence of procollagen alpha 2(I) mRNA in chemically transformed rat liver epithelial cells. J Biol Chem. 1984;259:1401–4. [PubMed] [Google Scholar]
- 16.Smith BD, Niles R. Characterization of collagen synthesized by normal and chemically transformed rat liver epithelial cell lines. Biochemistry. 1980;19:1820–5. doi: 10.1021/bi00550a014. [DOI] [PubMed] [Google Scholar]
- 17.DeClerck YA, Bomann ET, Spengler BA, Biedler JL. Differential collagen biosynthesis by human neuroblastoma cell variants. Cancer Res. 1987;47:6505–10. [PubMed] [Google Scholar]
- 18.Little CD, Church RL, Miller RA, Ruddle FH. Procollagen and collagen produced by a teratocarcinoma-derived cell line, TSD4: evidence for a new molecular form of collagen. Cell. 1977;10:287–95. doi: 10.1016/0092-8674(77)90222-7. [DOI] [PubMed] [Google Scholar]
- 19.Minafra S, Luparello C, Rallo F, Pucci-Minafra I. Collagen biosynthesis by a breast carcinoma cell strain and biopsy fragments of the primary tumour. Cell Biol Int Rep. 1988;12:895–905. doi: 10.1016/0309-1651(88)90053-7. [DOI] [PubMed] [Google Scholar]
- 20.Rupard JH, Dimari SJ, Damjanov I, Haralson MA. Synthesis of type I homotrimer collagen molecules by cultured human lung adenocarcinoma cells. Am J Pathol. 1988;133:316–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Ishikoh AU, Hayashi A, Tokimitsu I, Tajima S, Nishikawa T. Coordinate modulation of melanogenesis and type I trimer collagen secretion by type I collagen substratum during reversible conversion between melanotic and amelanotic cells in mouse B16 melanoma. J Biochem. 1994;116:610–4. doi: 10.1093/oxfordjournals.jbchem.a124568. [DOI] [PubMed] [Google Scholar]
- 22.Schillaci R, Luparello C, Minafra S. Type I and I-trimer collagens as substrates for breast carcinoma cells in culture. Effect on growth rate, morphological appearance and actin organization. Eur J Cell Biol. 1989;48:135–41. [PubMed] [Google Scholar]
- 23.Luparello C, Sheterline P, Pucci-Minafra I, Minafra S. A comparison of spreading and motility behaviour of 8701-BC breast carcinoma cells on type I, I-trimer and type V collagen substrata. Evidence for a permissive effect of type I-trimer collagen on cell locomotion. J Cell Sci. 1991;100(Pt 1):179–85. doi: 10.1242/jcs.100.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan AS, Meyers DF, Page RC, Welgus HG. Action of mammalian collagenases on type I trimer collagen. Coll Relat Res. 1984;4:289–96. doi: 10.1016/s0174-173x(84)80036-9. [DOI] [PubMed] [Google Scholar]
- 25.Makareeva E, Cabral WA, Marini JC, Leikin S. Molecular mechanism of alpha 1(I)-osteogenesis imperfecta/Ehlers-Danlos syndrome: unfolding of an N-anchor domain at the N-terminal end of the type I collagen triple helix. J Biol Chem. 2006;281:6463–70. doi: 10.1074/jbc.M511830200. [DOI] [PubMed] [Google Scholar]
- 26.Kuznetsova NV, McBride DJ, Leikin S. Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of alpha2(I) chain in osteogenesis imperfecta murine. J Mol Biol. 2003;331:191–200. doi: 10.1016/s0022-2836(03)00715-0. [DOI] [PubMed] [Google Scholar]
- 27.Schwarze U, Hata R, McKusick VA, et al. Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am J Hum Genet. 2004;74:917–30. doi: 10.1086/420794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung L, Dinakarpandian D, Yoshida N, et al. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. Embo J. 2004;23:3020–30. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkedal-Hansen H, Yamada S, Windsor J, et al. Matrix metalloproteinases. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb1008s40. Chapter 10:Unit 10 8. [DOI] [PubMed] [Google Scholar]
- 30.Makareeva E, Mertz EL, Kuznetsova NV, et al. Structural heterogeneity of type I collagen triple helix and its role in osteogenesis imperfecta. J Biol Chem. 2008;283:4787–98. doi: 10.1074/jbc.M705773200. [DOI] [PubMed] [Google Scholar]
- 31.Chipman SD, Sweet HO, McBride DJ, et al. Defective proα2(I) collagen synthesis in a recessive mutation in mice: A model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90:1701–5. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghersi G, La Fiura AM, Minafra S. Direct adhesion to type I and homotrimer collagens by breast carcinoma and embryonic epithelial cells in culture: a comparative study. Eur J Cell Biol. 1989;50:279–84. [PubMed] [Google Scholar]
- 33.Misof K, Landis WJ, Klaushofer K, Fratzl P. Collagen from the osteogenesis imperfecta mouse model (oim) shows reduced resistance against tensile stress. J Clin Invest. 1997;100:40–5. doi: 10.1172/JCI119519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han S, McBride DJ, Losert W, Leikin S. Segregation of type I collagen homo- and heterotrimers in fibrils. J Mol Biol. 2008;383:122–32. doi: 10.1016/j.jmb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander NR, Branch KM, Parekh A, et al. Extracellular matrix rigidity promotes invadopodia activity. Curr Biol. 2008;18:1295–9. doi: 10.1016/j.cub.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostic A, Lynch CD, Sheetz MP. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLoS One. 2009;4:e6361. doi: 10.1371/journal.pone.0006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167–74. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beare AH, O’Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–63. doi: 10.1046/j.1523-1747.2003.12019.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Wu H, Byrne M, Jeffrey J, Krane S, Jaenisch R. A targeted mutation at the known collagenase cleavage site in mouse type I collagen impairs tissue remodeling. J Cell Biol. 1995;130:227–37. doi: 10.1083/jcb.130.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao W, Byrne MH, Wang Y, Krane SM. Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J Clin Invest. 2000;106:941–9. doi: 10.1172/JCI10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brodeur AC, Wirth DA, Franklin CL, Reneker LW, Miner JH, Phillips CL. Type I collagen glomerulopathy: postnatal collagen deposition follows glomerular maturation. Kidney Int. 2007;71:985–93. doi: 10.1038/sj.ki.5002173. [DOI] [PubMed] [Google Scholar]
- 43.Barbolina MV, Stack MS. Membrane type 1-matrix metalloproteinase: substrate diversity in pericellular proteolysis. Semin Cell Dev Biol. 2008;19:24–33. doi: 10.1016/j.semcdb.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin KY, Lu D, Hung CF, et al. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res. 2007;67:1832–41. doi: 10.1158/0008-5472.CAN-06-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sengupta PK, Smith EM, Kim K, Murnane MJ, Smith BD. DNA hypermethylation near the transcription start site of collagen alpha2(I) gene occurs in both cancer cell lines and primary colorectal cancers. Cancer Res. 2003;63:1789–97. [PubMed] [Google Scholar]
- 46.Leheup BP, Federspiel SJ, Guerry-Force ML, et al. Extracellular matrix biosynthesis by cultured fetal rat lung epithelial cells. I. Characterization of the clone and the major genetic types of collagen produced. Lab Invest. 1989;60:791–807. [PubMed] [Google Scholar]
- 47.Mayne R, Vail MS, Miller EJ. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2′-deoxyuridine. Proc Natl Acad Sci U S A. 1975;72:4511–5. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diduch DR, Coe MR, Joyner C, Owen ME, Balian G. Two cell lines from bone marrow that differ in terms of collagen synthesis, osteogenic characteristics, and matrix mineralization. J Bone Joint Surg Am. 1993;75:92–105. doi: 10.2106/00004623-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Haralson MA, Jacobson HR, Hoover RL. Collagen polymorphism in cultured rat kidney mesangial cells. Lab Invest. 1987;57:513–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.