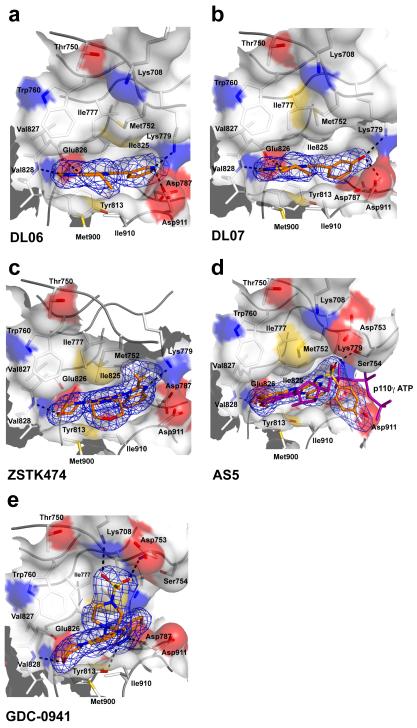
Figure 3.
The flat inhibitors DL06, DL07, ZSTK474, AS5 and GDC-0941 are multi- to pan-selective class I PI3K inhibitors that do not induce the opening of the “specificity” pocket. Shown are the binding modes of DL06 (a), DL07 (b), ZSTK474 (c), AS5 (d) and GDC-0941 (e) in the active site of p110δ. Met752 is in its “in” position for all these compounds. For panel (d), the structure of the p110γ/ATP complex (PDB entry 1e8x) was superimposed on the Cα-backbone of p110δ to show the proximity of the sulfonyl group of AS5 to the alpha phosphate group of ATP (purple). This sulfonyl group is a hydrogen bond acceptor to Ser754 located in the P-loop of p110δ. (e) GDC-0941 is a pan-class IA PI3K inhibitor that (like AS15) interacts with residues outside the active site. GDC-0941 occupies the “adenine” pocket and the “affinity” pocket within the active site of p110δ and engages there in hydrogen bonds with Val828, Tyr813 and Asp787. Additionally, the substituted piperazine group of GDC-0941 extends out of the ATP-binding site where its methylsulfonyl moiety acts as a hydrogen bond acceptor for Asp753 of the P-loop and Lys708 at the beginning of kα2. The contouring level of the 2mFo-DFc electron densities is 1σ for each compound.