Table 1.
Reaction Optimization for Enantioselective Bicyclization.a
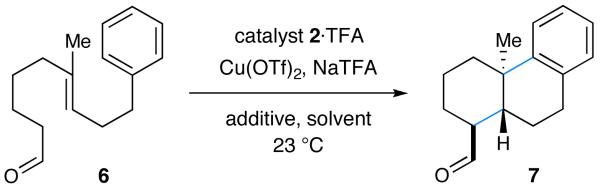 |
|||||
|---|---|---|---|---|---|
| entry | catalyst | additiveb | solvent | yield (%)c | ee (%)d |
| 1 | 2a, Ar = Ph (20 mol%) | none | MeCN | 11 | 34 |
| 2 | 2a, Ar = Ph (20 mol%) | TFA | MeCH | 16 | 35 |
| 3e | 2a, Ar = Ph (20 mol%) | TFA | MeCH | 42 | 42 |
| 4e | 2b, Ar = 1-Np (20 mol%) | TFA | MeCH | 56 | 74 |
| 5e | 2b, Ar = 1-Np (20 mol%) | TFA | i-PrCN/DMEf | 54 | 87 |
| 6e | 2b, Ar = 1-Np (30 mol%) | TFA | i-PrCN/DMEf | 70 | 87 |
Reactions peformed on a 0.20 mmol scale using 2.5 equiv of Cu(OTf)2 and 2.0 equiv of NaTFA.
3.0 equiv of TFA used.
Isolated yield.
ee determined by chiral HPLC analysis.
Slow addition of oxidant and base as a solution in MeCN or i-PrCN.
3:2 i-PrCN/DME (0.08 M).
