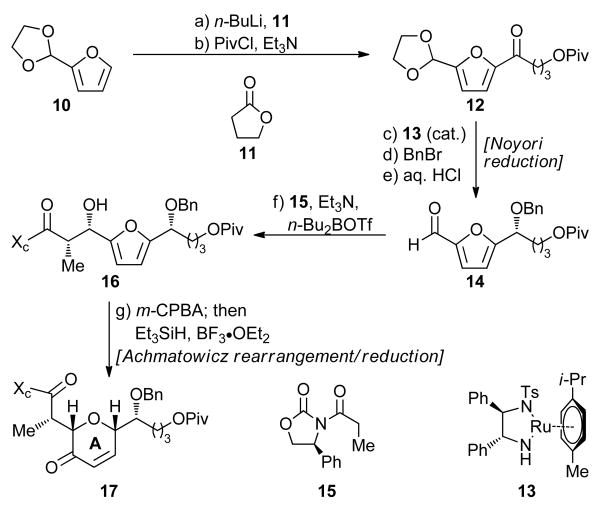
Scheme 1. Furan-based Synthesis of A Ring Building Block 17a.
aReagents and conditions: (a) n-BuLi (2.5 M in hexanes, 1.0 equiv), THF, −78 °C, 15 min; then 11 (1.0 equiv), −78 °C, 2.5 h, 62% plus 20% recovered starting material (10); (b) PivCl (1.2 equiv), Et3N (3.0 equiv), DMAP (0.1 equiv), CH2Cl2, 25 °C, 20 min, 94%; (c) catalyst 13 (0.01 equiv), n-Bu4NCl (0.3 equiv), HCO2Na (10.0 equiv), CH2Cl2:H2O (1:1), 25 °C, 48 h, 97% (94% ee by Naproxen® ester NMR spectroscopic analysis); (d) BnBr (2.5 equiv), n-Bu4NI (0.5 equiv), NaH (60% in mineral oil, 4.0 equiv), THF, 0 → 25 °C, 16 h, quant.; (e) 2.0 M aq. HCl:THF (1:2), 25 °C, quant.; (f) 15 (1.0 equiv), n-Bu2BOTf (1.0 M in CH2Cl2, 1.2 equiv), Et3N (1.3 equiv), −78 → 0 °C, CH2Cl2, 45 min; then 14, −78 → 0 °C, 4.5 h, 98%; (g) m-CPBA (1.2 equiv), CH2Cl2, 0 → 25 °C 2.5 h; then Et3SiH (2.0 equiv), BF3•OEt2 (2.0 equiv), −50 → −10 °C, 20 min, 75%.