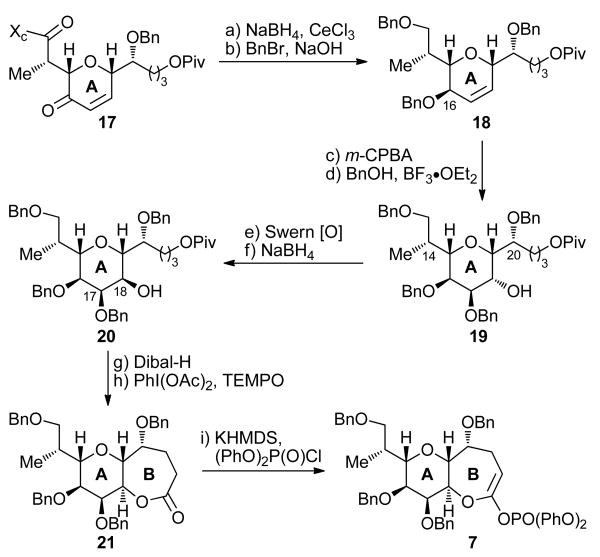
Scheme 2. Completion of the Synthesis of AB Ring System 7a.
aReagents and conditions: (a) NaBH4 (4.0 equiv), CeCl3 (2.0 equiv), CH2Cl2:MeOH (1:1), −30 → −10 °C, 15 min, 80%; (b) BnBr (25 equiv), n-Bu4NI (0.75 equiv), NaOH (25% aq.):PhMe (1:1), 25 °C, 48 h, 91%; (c) m-CPBA (3.0 equiv), CH2Cl2, 25 °C, 48 h, 71% (5:1 dr); (d) BnOH (2.5 equiv), BF3•OEt2, CH2Cl2, 25 °C, 18 h, 91%; (e) (COCl)2 (2.0 equiv), DMSO (4.0 equiv), CH2Cl2, −78 °C, 2 h; then Et3N (6.0 equiv), 0 °C, 1 h; (f) NaBH4 (2.0 equiv), MeOH, −78 °C, 45 min, 64% over the two steps; (g) Dibal-H (1.0 M in CH2Cl2, 2.5 equiv), CH2Cl2, −78 °C, 1 h, 83%; (h) PhI(OAc)2 (5.0 equiv), TEMPO (0.2 equiv), CH2Cl2, 25 °C, 48 h, 92%; (i) (PhO)2P(O)Cl (10.0 equiv), KHMDS (0.5 M in PhMe, 3.0 equiv), THF:HMPA (10:1), −78 °C, 30 min, 97%.