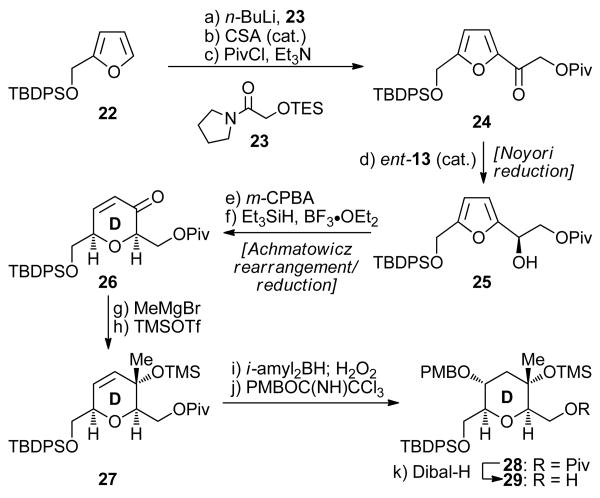
Scheme 3. Furan-Based Synthesis of D Ring Intermediate 29a.
aReagents and conditions: (a) 22 (1.2 equiv), n-BuLi (2.5 M in hexanes, 1.2 equiv), THF, −78 → 0 °C, 1 h; then 23 (1.0 equiv), −78 → 0 °C, 1 h, 84%; (b) CSA (0.1 equiv), CH2Cl2:MeOH (5:1), 25 °C, 1 h, 97%; (c) PivCl (1.2 equiv), Et3N (2.0 equiv), CH2Cl2, 25 °C, 12 h, 93%; (d) ent-13 (0.02 equiv), n-Bu4NCl (0.3 equiv), HCO2Na (10.0 equiv), CH2Cl2:H2O (1:1), 25 °C, 24 h, 94% (≥95% ee by Naproxen® ester NMR spectroscopic analysis); (e) m-CPBA (1.2 equiv), CH2Cl2, 0 → 25 °C, 2 h; (f) Et3SiH (3.0 equiv), BF3•OEt2 (2.0 equiv), CH2Cl2, −78 → −20 °C, 3 h, 74% over the two steps; (g) MeMgBr (2.0 equiv), THF, −78 °C, 2 h, 80%; (h) TMSOTf (1.5 equiv), Et3N (2.5 equiv), CH2Cl2, −78 °C, 1 h, 98%; (i) diisoamylborane (4.0 equiv), THF, −78 → 0 °C, 72 h; then H2O2 (35% aq., 40 equiv), NaOH (1.0 M aq., 80 equiv), 25 °C, 5 h, 75%; (j) PMBOC(NH)CCl3 (2.0 equiv), La(OTf)3 (0.05 equiv), PhMe, 25 °C, 3 h, 96%; (k) Dibal-H (2.2 equiv), CH2Cl2, −78 °C, 84%.