Scheme 4. Completion of the Synthesis of the DE Ring Coupling Partner 8a.
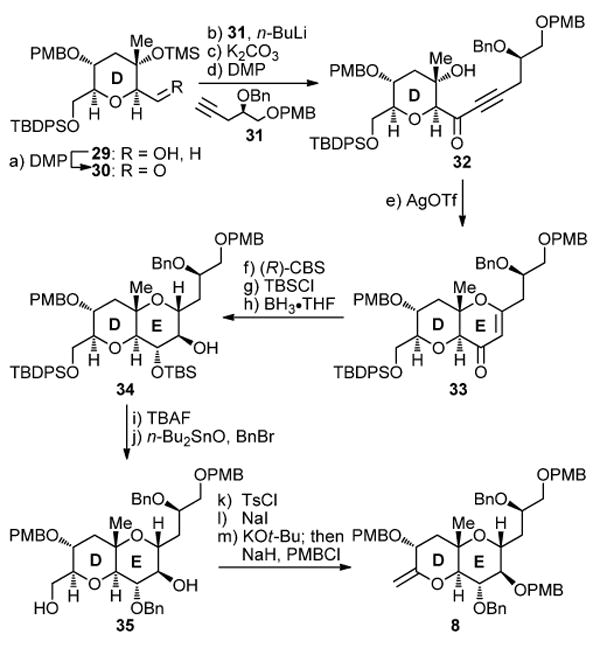
aReagents and conditions: (a) DMP (1.5 equiv), NaHCO3 (5.0 equiv), CH2Cl2, 25 °C, 1 h; (b) 31 (2.5 equiv), n-BuLi (2.5 M in hexanes, 2.5 equiv), THF, −78 → −40 °C, 10 min; then 30 (1.0 equiv), −78 → −50 °C, 1.5 h, 86% over the two steps; (c) K2CO3 (5.0 equiv), MeOH, 25 °C, 30 min, 99%; (d) DMP (1.5 equiv), CH2Cl2, 25 °C, 1 h, 91%; (e) AgOTf (0.9 equiv), CH2Cl2, −40 °C, 20 h, 76%; (f) (R)-CBS (1.0 M in PhMe, 1.5 equiv), BH3•THF (1.0 M in THF, 1.5 equiv), PhMe, −50 → −20 °C, 1 h, 93%; (g) TBSCl (6.0 equiv), imid. (10.0 equiv), CH2Cl2, 25 °C, 1 h, 98%; (h) BH3•THF (1.0 M in THF, 10 equiv), THF, 0 °C, 18 h; then H2O2 (35% aq., 100 equiv), NaOH (1.0 M aq., 200 equiv), 25 °C, 6 h, 54%; (i) TBAF (1.0 M in THF, 5.0 equiv), THF, 25 °C, 16 h, quant.; (j) n-Bu2SnO (1.0 equiv), PhMe, 110 °C, 12 h; then BnBr (1.5 equiv), n-Bu4NI (1.0 equiv), 100 °C, 4.5 h, 85%; (k) TsCl (3.0 equiv), Et3N (6.0 equiv), DMAP (0.1 equiv), CH2Cl2, 25 → 45 °C, 20 h; (l) NaI (10.0 equiv), DME, 85 °C, 7 h, 89% over the two steps; (m) KOt-Bu (12 equiv), THF, 0 °C, 16 h; then PMBCl (5.0 equiv), NaH (60% suspension in mineral oil, 10.0 equiv), n-Bu4NI (0.5 equiv), 25 °C, 36 h, 88%.
