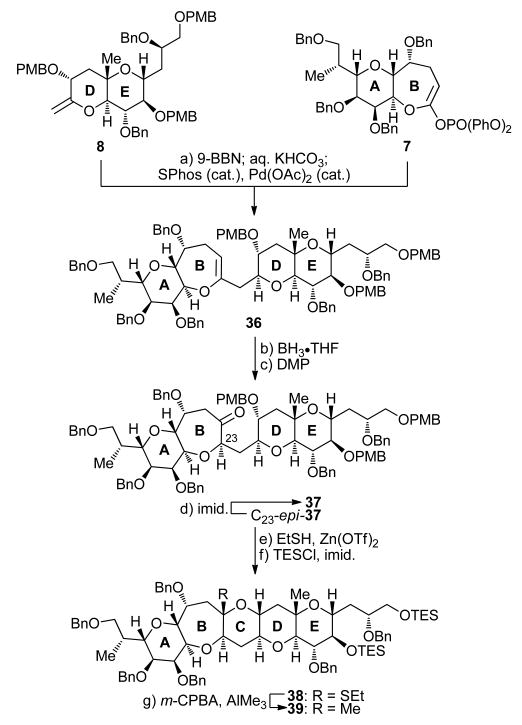
Scheme 5. Synthesis of ABCDE Ring System 39a.
aReagents and conditions: (a) 8 (1.5 equiv), 9-BBN (0.5 M in THF, 4.5 equiv), THF, 25 °C, 4 h; then KHCO3 (0.5 M aq., 13.5 equiv), 25 °C, 20 min; then 7, SPhos (0.3 equiv), Pd(OAc)2 (0.15 equiv), 25 °C, 72 h, 90%; (b) BH3•THF (1.0 M in THF, 10.0 equiv), THF, 0 °C, 18 h; then H2O2 (35% aq., 100 equiv), NaOH (1.0 M aq., 200 equiv), 25 °C, 6 h, 70% (α-diastereomer) plus 15% (β-diastereomer); (c) DMP (4.0 equiv), CH2Cl2, 0 → 25 °C, 2 h, 97% for 37, 84% for C23-epi-37; (d) imid. (200 equiv), PhMe, 105 °C, 120 h, 65% plus 26% recovered starting material (C23-epi-37); (e) 37 (1.0 equiv), Zn(OTf)2 (5.0 equiv), EtSH:CH2Cl2 (1:5), 25 °C, 20 h; (f) TESCl (10.0 equiv), imid. (20 equiv), CH2Cl2, 25 °C, 1 h, 91% over the two steps; (g) m-CPBA (2.5 equiv), CH2Cl2, 0 °C, 30 min; then AlMe3 (2.0 M in hexanes, 5.0 equiv), 0 °C, 30 min, 94%.