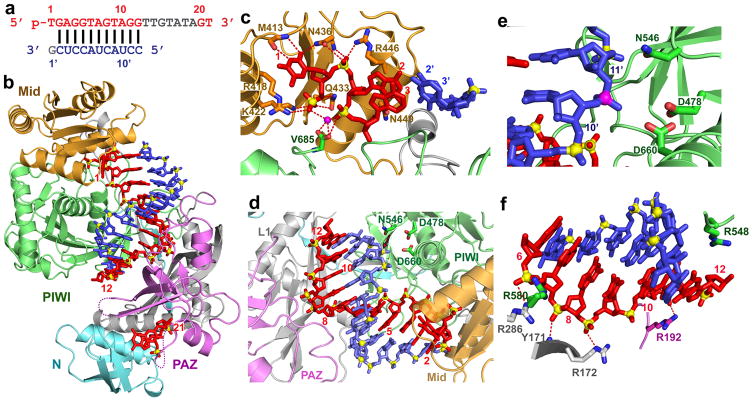
Figure 1. Crystal structure of T. thermophilus Ago containing N546 catalytic mutant bound to 5′-phosphorylated 21-nt guide DNA and 12-nt target RNA.
a, Sequence of the guide DNA-target RNA duplex. The traceable segments of the bases of the guide DNA and target RNA in the structure of the ternary complex are shown in red and blue, respectively. Disordered segments of the bases on both strands that cannot be traced are shown in grey. b, A view of the 2.6 Å crystal structure of the Ago ternary complex. The Ago protein is color-coded by domains (N in cyan, PAZ in magenta, Mid in orange and PIWI in green) and linkers (L1 and L2 in grey). The bound 21-nt guide DNA is in red and traced for bases 1–12 and 20–21, whereas the bound 12-nt target RNA is in blue and traced for bases 2′–12′. Backbone phosphorus atoms are in yellow. Both ends of the bound guide DNA are anchored in this ternary complex. c, An expanded view of the ternary complex highlighting the alignment of guide DNA (1–3) and target RNA (2′–3′), where the bases of the 1–2 step of the guide strand are splayed apart. Note the intermolecular hydrogen-bonding of the Watson-crick edge of T1 with the backbone amide carbonyl of M413 and side chain of N436, as well as the positioning of phosphate 1 of the guide strand in the Mid binding pocket. A Mg2+ cation (purple) coordinates to phosphates 1 and 3 of the guide strand, as well as to an inserted carboxylate of V685 from the C-terminus of the protein. d, An expanded view of the ternary complex highlighting the guide DNA (1–12) - target RNA (2′–12′) duplex, together with the catalytic residues (D478, D660 and N546 mutant) of the RNase H fold of the PIWI domain. The scissile phosphate group at the 10–11 step of the target RNA is indicated by a red arrow. e, An expanded view highlighting the positioning of the backbone phosphate linking the 10–11 step (phosphorus colored in magenta) of the target RNA relative to the catalytic residues (D478, D660 and N546 mutant) in the ternary complex. f, Positioning of the side chain of R548 relative to the guide DNA (6 to 12) - target RNA (6′ to 12′) duplex. Note the intermolecular contacts between the sugar-phosphate backbone of the guide strand and side chains of the protein in the ternary complex.