Abstract
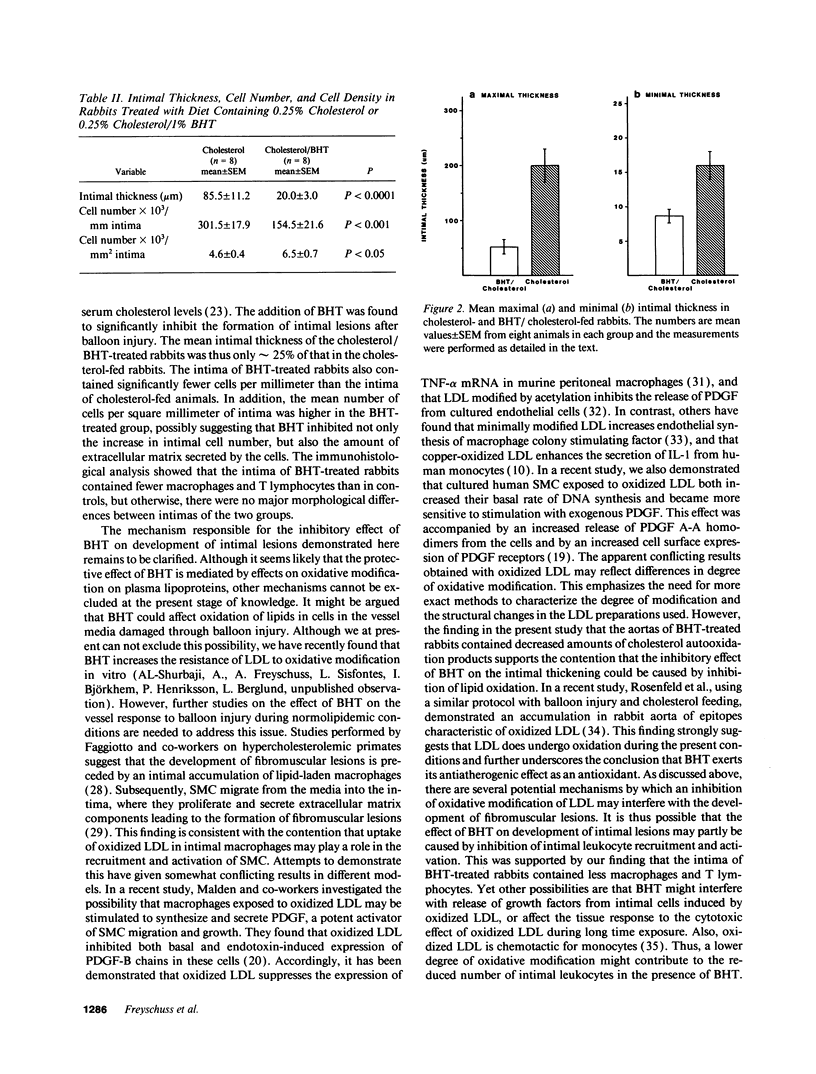
The effect of the antioxidant butylated hydroxytoluene (BHT) on the accumulation of intimal smooth muscle cells (SMC) and development of intimal thickening after balloon catheter injury of the aorta were studied in rabbits with dietary-induced hyperlipidemia. Two sets of New Zealand White rabbits (eight rabbits in each group) were fed either 0.25% cholesterol or 0.25% cholesterol/1% BHT for a total of 6 wk. Serum lipid levels did not differ between the two groups. 3 wk after the start of the study, a balloon injury of the aorta was performed, after which the rabbits were kept on their respective diets for another 3 wk. After this period of time, the rabbits were killed and their aortas were investigated. The BHT-treated rabbits had only one fourth of the intimal thickness (P < 0.0001) and half the number of SMC/mm intima (P < 0.001), as compared to the rabbits fed only cholesterol. There was also a lower number of macrophages in the BHT-treated group. T lymphocytes were present in the intima of cholesterol-fed rabbits, whereas no such cells could be identified in the BHT-fed animals. There were significantly lower levels of autooxidation products of cholesterol (7-oxocholesterol, cholesterol-5,6-epoxide, and 7 beta-hydroxycholesterol) in the aortas of BHT-treated rabbits, P < 0.001. In conclusion, the antioxidant BHT effectively inhibited the accumulation of intimal SMC and the development of intimal thickening of the aorta in hypercholesterolemic rabbits after a balloon catheter-induced injury. These results indicate that antioxidants may modify intimal response to injury.
Full text
PDF


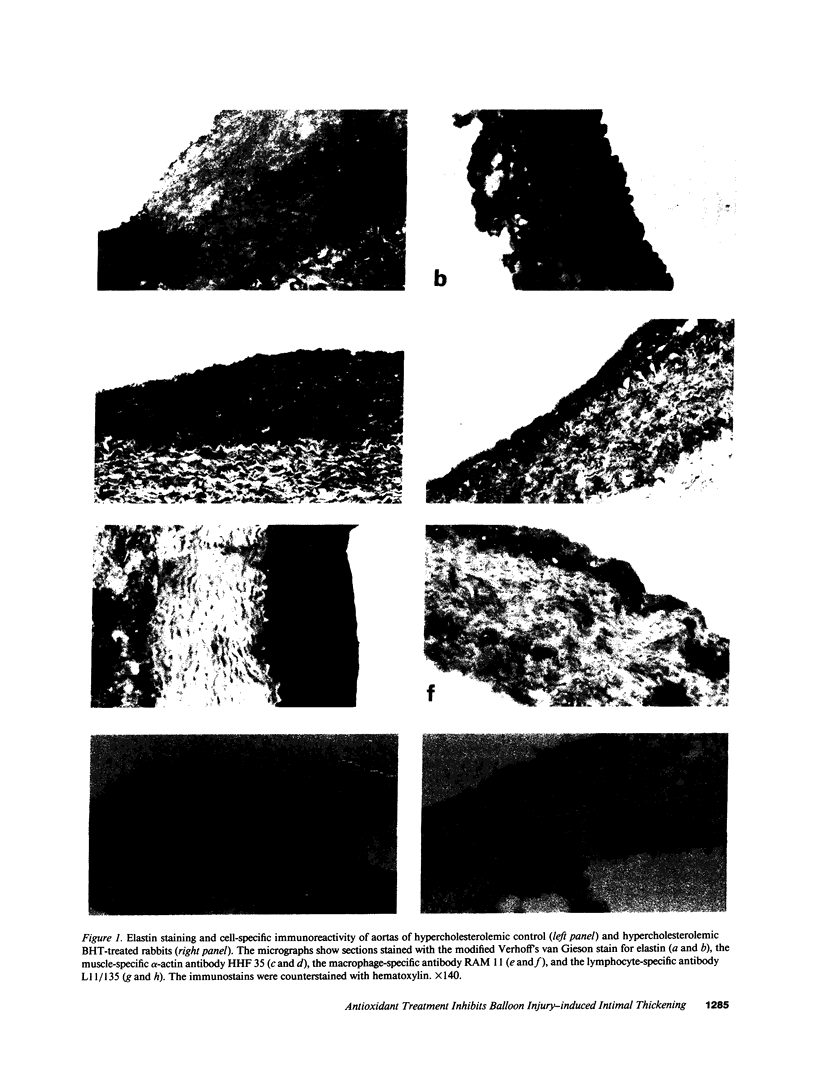


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Benditt E. P. sis (platelet-derived growth factor B chain) gene transcript levels are elevated in human atherosclerotic lesions compared to normal artery. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1099–1103. doi: 10.1073/pnas.84.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berliner J. A., Territo M. C., Sevanian A., Ramin S., Kim J. A., Bamshad B., Esterson M., Fogelman A. M. Minimally modified low density lipoprotein stimulates monocyte endothelial interactions. J Clin Invest. 1990 Apr;85(4):1260–1266. doi: 10.1172/JCI114562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Breuer O., Björkhem I. Simultaneous quantification of several cholesterol autoxidation and monohydroxylation products by isotope-dilution mass spectrometry. Steroids. 1990 Apr;55(4):185–192. doi: 10.1016/0039-128x(90)90109-o. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catignani G. L., Bieri J. G. Simultaneous determination of retinol and alpha-tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983 Apr;29(4):708–712. [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Stewart-Lee A. L., Anggård E. E. Arterial response to mechanical injury: balloon catheter de-endothelialization. Atherosclerosis. 1992 Feb;92(2-3):89–104. doi: 10.1016/0021-9150(92)90268-l. [DOI] [PubMed] [Google Scholar]
- Fox P. L., DiCorleto P. E. Modified low density lipoproteins suppress production of a platelet-derived growth factor-like protein by cultured endothelial cells. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4774–4778. doi: 10.1073/pnas.83.13.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård J., Haegerstrand A., Gidlund M., Nilsson J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis. 1991 Oct;90(2-3):119–126. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- Frostegård J., Nilsson J., Haegerstrand A., Hamsten A., Wigzell H., Gidlund M. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Feb;87(3):904–908. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård J., Wu R., Giscombe R., Holm G., Lefvert A. K., Nilsson J. Induction of T-cell activation by oxidized low density lipoprotein. Arterioscler Thromb. 1992 Apr;12(4):461–467. doi: 10.1161/01.atv.12.4.461. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Ma G. P., Chisolm G. M. Oxidized low density lipoprotein suppresses the expression of tumor necrosis factor-alpha mRNA in stimulated murine peritoneal macrophages. J Immunol. 1990 Mar 15;144(6):2343–2350. [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Kaluzny M. A., Duncan L. A., Merritt M. V., Epps D. E. Rapid separation of lipid classes in high yield and purity using bonded phase columns. J Lipid Res. 1985 Jan;26(1):135–140. [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malden L. T., Chait A., Raines E. W., Ross R. The influence of oxidatively modified low density lipoproteins on expression of platelet-derived growth factor by human monocyte-derived macrophages. J Biol Chem. 1991 Jul 25;266(21):13901–13907. [PubMed] [Google Scholar]
- Nilsson J. Growth factors and the pathogenesis of atherosclerosis. Atherosclerosis. 1986 Dec;62(3):185–199. doi: 10.1016/0021-9150(86)90093-6. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Thyberg J., Heldin C. H. Arterial smooth muscle cells in primary culture produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4418–4422. doi: 10.1073/pnas.82.13.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Khoo J. C., Miller E., Parthasarathy S., Palinski W., Witztum J. L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991 Jan;87(1):90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Shimokado K., Raines E. W., Madtes D. K., Barrett T. B., Benditt E. P., Ross R. A significant part of macrophage-derived growth factor consists of at least two forms of PDGF. Cell. 1985 Nov;43(1):277–286. doi: 10.1016/0092-8674(85)90033-9. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stiko-Rahm A., Hultgårdh-Nilsson A., Regnström J., Hamsten A., Nilsson J. Native and oxidized LDL enhances production of PDGF AA and the surface expression of PDGF receptors in cultured human smooth muscle cells. Arterioscler Thromb. 1992 Sep;12(9):1099–1109. doi: 10.1161/01.atv.12.9.1099. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Smith K. M., Williams L. T., Schwartz S. M., Gordon D. Platelet-derived growth factor mRNA detection in human atherosclerotic plaques by in situ hybridization. J Clin Invest. 1988 Sep;82(3):1134–1143. doi: 10.1172/JCI113671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]













