Figure 1.
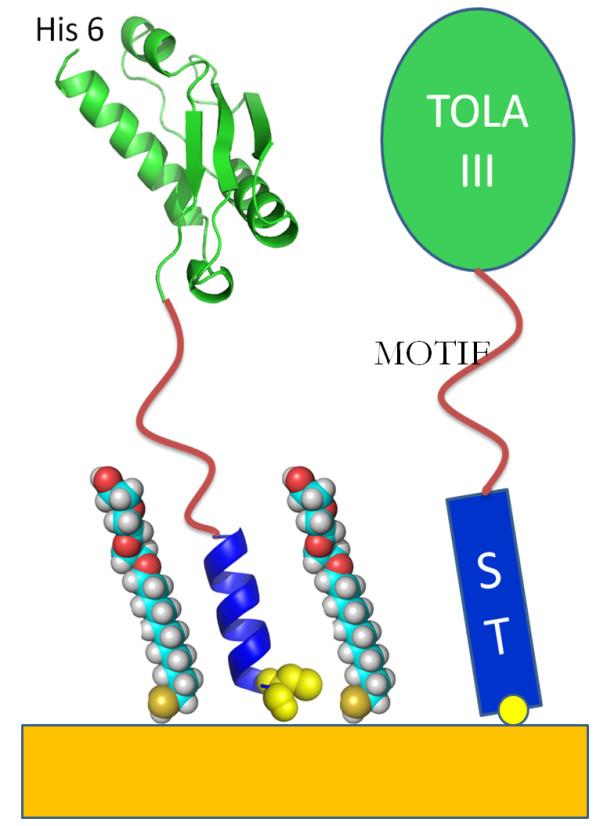
The design of the Tol switch tag system, a molecular image (left) and a schematic (right). The protein starts at the N-terminus with a 6 His tag for easy purification followed by a FLAG-tag sequence to aid detection. The TolAIII domain which comes next provides solubility and drives high levels of expression for fusion proteins attached to its C terminus [10]. The fusion sequence is inserted between the TolA and the switch tag, which is found at the C terminus, by use of a multiple cloning site in the plasmid [8]. The TolA domain can be removed by specific proteolytic cleavage but in most cases it is left in place since it stabilises the fusion construct. The switch tag is shown here as a helix whose hydrophobic properties allow co assembly with alkane thiol SAM, in solution it is a water soluble random coil.
